Identification of Surface Epitopes Associated with Protection against Highly Immune-Evasive VlsE-Expressing Lyme Disease Spirochetes
- PMID: 29866906
- PMCID: PMC6056884
- DOI: 10.1128/IAI.00182-18
Identification of Surface Epitopes Associated with Protection against Highly Immune-Evasive VlsE-Expressing Lyme Disease Spirochetes
Abstract
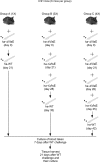
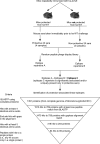
The tick-borne pathogen Borrelia burgdorferi is responsible for approximately 300,000 Lyme disease (LD) cases per year in the United States. Recent increases in the number of LD cases, in addition to the spread of the tick vector and a lack of a vaccine, highlight an urgent need for designing and developing an efficacious LD vaccine. Identification of protective epitopes that could be used to develop a second-generation (subunit) vaccine is therefore imperative. Despite the antigenicity of several lipoproteins and integral outer membrane proteins (OMPs) on the B. burgdorferi surface, the spirochetes successfully evade antibodies primarily due to the VlsE-mediated antigenic variation. VlsE is thought to sterically block antibody access to protective epitopes of B. burgdorferi However, it is highly unlikely that VlsE shields the entire surface epitome. Thus, identification of subdominant epitope targets that induce protection when they are made dominant is necessary to generate an efficacious vaccine. Toward the identification, we repeatedly immunized immunocompetent mice with live-attenuated VlsE-deleted B. burgdorferi and then challenged the animals with the VlsE-expressing (host-adapted) wild type. Passive immunization and Western blotting data suggested that the protection of 50% of repeatedly immunized animals against the highly immune-evasive B. burgdorferi was antibody mediated. Comparison of serum antibody repertoires identified in protected and nonprotected animals permitted the identification of several putative epitopes significantly associated with the protection. Most linear putative epitopes were conserved between the main pathogenic Borrelia genospecies and found within known subdominant regions of OMPs. Currently, we are performing immunization studies to test whether the identified protection-associated epitopes are protective for mice.
Keywords: Borrelia burgdorferi; Lyme disease; VlsE; epitopes; immunization; protection; surface antigens; vls locus.
Copyright © 2018 American Society for Microbiology.
Figures





Similar articles
-
Delineating Surface Epitopes of Lyme Disease Pathogen Targeted by Highly Protective Antibodies of New Zealand White Rabbits.Infect Immun. 2019 Jul 23;87(8):e00246-19. doi: 10.1128/IAI.00246-19. Print 2019 Aug. Infect Immun. 2019. PMID: 31085705 Free PMC article.
-
Antibody Response to Lyme Disease Spirochetes in the Context of VlsE-Mediated Immune Evasion.Infect Immun. 2016 Dec 29;85(1):e00890-16. doi: 10.1128/IAI.00890-16. Print 2017 Jan. Infect Immun. 2016. PMID: 27799330 Free PMC article.
-
Variable VlsE is critical for host reinfection by the Lyme disease spirochete.PLoS One. 2013 Apr 8;8(4):e61226. doi: 10.1371/journal.pone.0061226. Print 2013. PLoS One. 2013. PMID: 23593438 Free PMC article.
-
Prevention of lyme disease: promising research or sisyphean task?Arch Immunol Ther Exp (Warsz). 2011 Aug;59(4):261-75. doi: 10.1007/s00005-011-0128-z. Epub 2011 Jun 3. Arch Immunol Ther Exp (Warsz). 2011. PMID: 21633917 Review.
-
vls Antigenic Variation Systems of Lyme Disease Borrelia: Eluding Host Immunity through both Random, Segmental Gene Conversion and Framework Heterogeneity.Microbiol Spectr. 2014 Dec;2(6):10.1128/microbiolspec.MDNA3-0038-2014. doi: 10.1128/microbiolspec.MDNA3-0038-2014. Microbiol Spectr. 2014. PMID: 26104445 Free PMC article. Review.
Cited by
-
Antiphospholipid autoantibodies in Lyme disease arise after scavenging of host phospholipids by Borrelia burgdorferi.J Clin Invest. 2022 Mar 15;132(6):e152506. doi: 10.1172/JCI152506. J Clin Invest. 2022. PMID: 35289310 Free PMC article.
-
Immunoinformatics-Based Proteome Mining to Develop a Next-Generation Vaccine Design against Borrelia burgdorferi: The Cause of Lyme Borreliosis.Vaccines (Basel). 2022 Aug 2;10(8):1239. doi: 10.3390/vaccines10081239. Vaccines (Basel). 2022. PMID: 36016127 Free PMC article.
-
Delineating Surface Epitopes of Lyme Disease Pathogen Targeted by Highly Protective Antibodies of New Zealand White Rabbits.Infect Immun. 2019 Jul 23;87(8):e00246-19. doi: 10.1128/IAI.00246-19. Print 2019 Aug. Infect Immun. 2019. PMID: 31085705 Free PMC article.
-
Meta-analysis of the Vmp-like sequences of Lyme disease Borrelia: evidence for the evolution of an elaborate antigenic variation system.Front Microbiol. 2024 Oct 10;15:1469411. doi: 10.3389/fmicb.2024.1469411. eCollection 2024. Front Microbiol. 2024. PMID: 39450289 Free PMC article.
-
New Zealand White Rabbits Effectively Clear Borrelia burgdorferi B31 despite the Bacterium's Functional vlsE Antigenic Variation System.Infect Immun. 2019 Jun 20;87(7):e00164-19. doi: 10.1128/IAI.00164-19. Print 2019 Jul. Infect Immun. 2019. PMID: 30988058 Free PMC article.
References
-
- Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. 2011. Lyme borreliosis in Europe. Euro Surveill 16(27):pii=19906 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId_19906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

