Candida albicans Cannot Acquire Sufficient Ethanolamine from the Host To Support Virulence in the Absence of De Novo Phosphatidylethanolamine Synthesis
- PMID: 29866908
- PMCID: PMC6056879
- DOI: 10.1128/IAI.00815-17
Candida albicans Cannot Acquire Sufficient Ethanolamine from the Host To Support Virulence in the Absence of De Novo Phosphatidylethanolamine Synthesis
Abstract
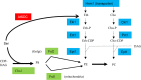
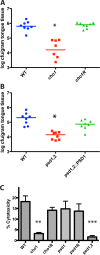
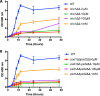
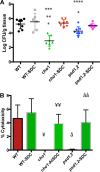
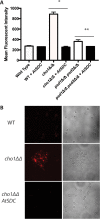
Candida albicans mutants for phosphatidylserine (PS) synthase (cho1ΔΔ) and PS decarboxylase (psd1ΔΔ psd2ΔΔ) are compromised for virulence in mouse models of systemic infection and oropharyngeal candidiasis (OPC). Both of these enzymes are necessary to synthesize phosphatidylethanolamine (PE) by the de novo pathway, but these mutants are still capable of growth in culture media, as they can import ethanolamine from media to synthesize PE through the Kennedy pathway. Given that the host has ethanolamine in its serum, the exact mechanism by which virulence is lost in these mutants is not clear. There are two competing hypotheses to explain their loss of virulence. (i) PE from the Kennedy pathway cannot substitute for de novo-synthesized PE. (ii) The mutants cannot acquire sufficient ethanolamine from the host to support adequate PE synthesis. These hypotheses can be simultaneously tested if ethanolamine availability is increased for Candida while it is inside the host. We accomplish this by transcomplementation of C. albicans with the Arabidopsis thaliana serine decarboxylase gene (AtSDC), which converts cytoplasmic serine to ethanolamine. Expression of AtSDC in either mutant restores PE synthesis, even in the absence of exogenous ethanolamine. AtSDC also restores virulence to cho1ΔΔ and psd1ΔΔ psd2ΔΔ strains in systemic and OPC infections. Thus, in the absence of de novo PE synthesis, C. albicans cannot acquire sufficient ethanolamine from the host to support virulence. In addition, expression of AtSDC restores PS synthesis in the cho1ΔΔ mutant, which may be due to causing PS decarboxylase to run backwards and convert PE to PS.
Keywords: Candida albicans; mice; oropharyngeal; phosphatidylethanolamine; phosphatidylserine; serine decarboxylase; virulence.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
Pathways That Synthesize Phosphatidylethanolamine Impact Candida albicans Hyphal Length and Cell Wall Composition through Transcriptional and Posttranscriptional Mechanisms.Infect Immun. 2020 Feb 20;88(3):e00480-19. doi: 10.1128/IAI.00480-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31792076 Free PMC article.
-
Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans.Mol Microbiol. 2010 Mar;75(5):1112-32. doi: 10.1111/j.1365-2958.2009.07018.x. Epub 2010 Feb 4. Mol Microbiol. 2010. PMID: 20132453
-
Arabidopsis serine decarboxylase mutants implicate the roles of ethanolamine in plant growth and development.Int J Mol Sci. 2012;13(3):3176-3188. doi: 10.3390/ijms13033176. Epub 2012 Mar 7. Int J Mol Sci. 2012. PMID: 22489147 Free PMC article.
-
PS, It's Complicated: The Roles of Phosphatidylserine and Phosphatidylethanolamine in the Pathogenesis of Candida albicans and Other Microbial Pathogens.J Fungi (Basel). 2018 Feb 20;4(1):28. doi: 10.3390/jof4010028. J Fungi (Basel). 2018. PMID: 29461490 Free PMC article. Review.
-
Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells.Biochim Biophys Acta. 2013 Mar;1831(3):543-54. doi: 10.1016/j.bbalip.2012.08.016. Epub 2012 Aug 29. Biochim Biophys Acta. 2013. PMID: 22960354 Review.
Cited by
-
Mapping the Substrate-Binding Sites in the Phosphatidylserine Synthase in Candida albicans.Front Cell Infect Microbiol. 2021 Dec 22;11:765266. doi: 10.3389/fcimb.2021.765266. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004345 Free PMC article.
-
Mucosal Infection with Unmasked Candida albicans Cells Impacts Disease Progression in a Host Niche-Specific Manner.Infect Immun. 2022 Dec 15;90(12):e0034222. doi: 10.1128/iai.00342-22. Epub 2022 Nov 14. Infect Immun. 2022. PMID: 36374100 Free PMC article.
-
Pathways That Synthesize Phosphatidylethanolamine Impact Candida albicans Hyphal Length and Cell Wall Composition through Transcriptional and Posttranscriptional Mechanisms.Infect Immun. 2020 Feb 20;88(3):e00480-19. doi: 10.1128/IAI.00480-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31792076 Free PMC article.
-
Structural insights into phosphatidylethanolamine formation in bacterial membrane biogenesis.Sci Rep. 2021 Mar 11;11(1):5785. doi: 10.1038/s41598-021-85195-5. Sci Rep. 2021. PMID: 33707636 Free PMC article.
-
Candida albicans rvs161Δ and rvs167Δ Endocytosis Mutants Are Defective in Invasion into the Oral Cavity.mBio. 2019 Nov 12;10(6):e02503-19. doi: 10.1128/mBio.02503-19. mBio. 2019. PMID: 31719181 Free PMC article.
References
-
- Hancock PJ, Epstein JB, Sadler GR. 2003. Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc 69:585–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

