Methylthioadenosine Suppresses Salmonella Virulence
- PMID: 29866910
- PMCID: PMC6105896
- DOI: 10.1128/IAI.00429-18
Methylthioadenosine Suppresses Salmonella Virulence
Abstract
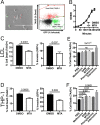
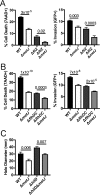
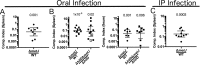
In order to deploy virulence factors at appropriate times and locations, microbes must rapidly sense and respond to various metabolite signals. Previously, we showed a transient elevation of the methionine-derived metabolite methylthioadenosine (MTA) concentration in serum during systemic Salmonella enterica serovar Typhimurium infection. Here we explored the functional consequences of increased MTA concentrations on S Typhimurium virulence. We found that MTA, but not other related metabolites involved in polyamine synthesis and methionine salvage, reduced motility, host cell pyroptosis, and cellular invasion. Further, we developed a genetic model of increased bacterial endogenous MTA production by knocking out the master repressor of the methionine regulon, metJ Like MTA-treated S Typhimurium, the ΔmetJ mutant displayed reduced motility, host cell pyroptosis, and invasion. These phenotypic effects of MTA correlated with suppression of flagellar and Salmonella pathogenicity island 1 (SPI-1) networks. S Typhimurium ΔmetJ had reduced virulence in oral and intraperitoneal infection of C57BL/6J mice independently of the effects of MTA on SPI-1. Finally, ΔmetJ bacteria induced a less severe inflammatory cytokine response in a mouse sepsis model. Together, these data indicate that exposure of S Typhimurium to MTA or disruption of the bacterial methionine metabolism pathway suppresses S Typhimurium virulence.
Keywords: SPI-1; Salmonella; flagellar motility; inflammation; metJ; metabolism; methionine salvage; methylthioadenosine; virulence regulation.
Copyright © 2018 Bourgeois et al.
Figures





Similar articles
-
Differences in the expression of SPI-1 genes pathogenicity and epidemiology between the emerging Salmonella enterica serovar Infantis and the model Salmonella enterica serovar Typhimurium.J Infect Dis. 2019 Aug 9;220(6):1071-1081. doi: 10.1093/infdis/jiz235. J Infect Dis. 2019. PMID: 31062854
-
QseC mediates Salmonella enterica serovar typhimurium virulence in vitro and in vivo.Infect Immun. 2010 Mar;78(3):914-26. doi: 10.1128/IAI.01038-09. Epub 2009 Dec 22. Infect Immun. 2010. PMID: 20028809 Free PMC article.
-
Epigallocatechin gallate protects mice from Salmonella enterica ser. Typhimurium infection by modulating bacterial virulence through quorum sensing inhibition.Front Cell Infect Microbiol. 2024 Oct 16;14:1432111. doi: 10.3389/fcimb.2024.1432111. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39479281 Free PMC article.
-
Polyamines are required for virulence in Salmonella enterica serovar Typhimurium.PLoS One. 2012;7(4):e36149. doi: 10.1371/journal.pone.0036149. Epub 2012 Apr 30. PLoS One. 2012. PMID: 22558361 Free PMC article.
-
YeiE Regulates Motility and Gut Colonization in Salmonella enterica Serotype Typhimurium.mBio. 2021 Jun 29;12(3):e0368020. doi: 10.1128/mBio.03680-20. Epub 2021 Jun 8. mBio. 2021. PMID: 34098734 Free PMC article.
Cited by
-
Genome-wide phenotypic profiling of transcription factors and identification of novel targets to control the virulence of Vibrio vulnificus.Nucleic Acids Res. 2025 Jan 24;53(3):gkae1238. doi: 10.1093/nar/gkae1238. Nucleic Acids Res. 2025. PMID: 39704106 Free PMC article.
-
Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network.Front Cell Infect Microbiol. 2019 Jul 31;9:270. doi: 10.3389/fcimb.2019.00270. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31428589 Free PMC article. Review.
-
Skyline for Small Molecules: A Unifying Software Package for Quantitative Metabolomics.J Proteome Res. 2020 Apr 3;19(4):1447-1458. doi: 10.1021/acs.jproteome.9b00640. Epub 2020 Mar 26. J Proteome Res. 2020. PMID: 31984744 Free PMC article.
-
Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies.Heliyon. 2020 Sep 22;6(9):e05020. doi: 10.1016/j.heliyon.2020.e05020. eCollection 2020 Sep. Heliyon. 2020. PMID: 32995651 Free PMC article. Review.
-
Revisiting the methionine salvage pathway and its paralogues.Microb Biotechnol. 2019 Jan;12(1):77-97. doi: 10.1111/1751-7915.13324. Epub 2018 Oct 10. Microb Biotechnol. 2019. PMID: 30306718 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

