Multiscale understanding of tricalcium silicate hydration reactions
- PMID: 29867195
- PMCID: PMC5986785
- DOI: 10.1038/s41598-018-26943-y
Multiscale understanding of tricalcium silicate hydration reactions
Abstract
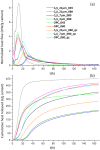
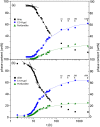
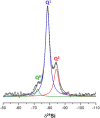
Tricalcium silicate, the main constituent of Portland cement, hydrates to produce crystalline calcium hydroxide and calcium-silicate-hydrates (C-S-H) nanocrystalline gel. This hydration reaction is poorly understood at the nanoscale. The understanding of atomic arrangement in nanocrystalline phases is intrinsically complicated and this challenge is exacerbated by the presence of additional crystalline phase(s). Here, we use calorimetry and synchrotron X-ray powder diffraction to quantitatively follow tricalcium silicate hydration process: i) its dissolution, ii) portlandite crystallization and iii) C-S-H gel precipitation. Chiefly, synchrotron pair distribution function (PDF) allows to identify a defective clinotobermorite, Ca11Si9O28(OH)2.8.5H2O, as the nanocrystalline component of C-S-H. Furthermore, PDF analysis also indicates that C-S-H gel contains monolayer calcium hydroxide which is stretched as recently predicted by first principles calculations. These outcomes, plus additional laboratory characterization, yielded a multiscale picture for C-S-H nanocomposite gel which explains the observed densities and Ca/Si atomic ratios at the nano- and meso- scales.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Le Chatelier, H. Recherches Expérimentales sur la Constitution des Mortiers Hydrauliques (Doctoral thesis, Faculté des Sciences de Paris, 1887).
-
- Taylor, H. F. W. Cement Chemistry (Thomas Telford, London, 1997)
-
- Bullard JW, et al. Mechanisms of Cement Hydration. Cem. Concr. Res. 2011;41:1208–1223. doi: 10.1016/j.cemconres.2010.09.011. - DOI
-
- Nugent MA, Brantley SL, Pantano CG. & Maurice, P. A. The influence of natural mineral coatings on feldspar weathering. Nature. 1998;395:588–591. doi: 10.1038/26951. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

