Force-Mediating Magnetic Nanoparticles to Engineer Neuronal Cell Function
- PMID: 29867315
- PMCID: PMC5962660
- DOI: 10.3389/fnins.2018.00299
Force-Mediating Magnetic Nanoparticles to Engineer Neuronal Cell Function
Abstract
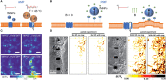
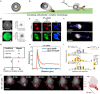
Cellular processes like membrane deformation, cell migration, and transport of organelles are sensitive to mechanical forces. Technically, these cellular processes can be manipulated through operating forces at a spatial precision in the range of nanometers up to a few micrometers through chaperoning force-mediating nanoparticles in electrical, magnetic, or optical field gradients. But which force-mediating tool is more suitable to manipulate cell migration, and which, to manipulate cell signaling? We review here the differences in forces sensation to control and engineer cellular processes inside and outside the cell, with a special focus on neuronal cells. In addition, we discuss technical details and limitations of different force-mediating approaches and highlight recent advancements of nanomagnetics in cell organization, communication, signaling, and intracellular trafficking. Finally, we give suggestions about how force-mediating nanoparticles can be used to our advantage in next-generation neurotherapeutic devices.
Keywords: cell communication; cell guidance; cell polarity; intracellular forces; nanomagnetics; nanoparticles; neurons.
Figures





Similar articles
-
Engineering cortical neuron polarity with nanomagnets on a chip.ACS Nano. 2015;9(4):3664-76. doi: 10.1021/nn505330w. Epub 2015 Apr 1. ACS Nano. 2015. PMID: 25801533
-
Modulating motility of intracellular vesicles in cortical neurons with nanomagnetic forces on-chip.Lab Chip. 2017 Feb 28;17(5):842-854. doi: 10.1039/c6lc01349j. Lab Chip. 2017. PMID: 28164203 Free PMC article.
-
Magnetic Nanotweezers for Interrogating Biological Processes in Space and Time.Acc Chem Res. 2018 Apr 17;51(4):839-849. doi: 10.1021/acs.accounts.8b00004. Epub 2018 Mar 28. Acc Chem Res. 2018. PMID: 29589897 Free PMC article. Review.
-
Parallelized Manipulation of Adherent Living Cells by Magnetic Nanoparticles-Mediated Forces.Int J Mol Sci. 2020 Sep 8;21(18):6560. doi: 10.3390/ijms21186560. Int J Mol Sci. 2020. PMID: 32911745 Free PMC article.
-
Intracellular mechanics: connecting rheology and mechanotransduction.Curr Opin Cell Biol. 2019 Feb;56:34-44. doi: 10.1016/j.ceb.2018.08.007. Epub 2018 Sep 22. Curr Opin Cell Biol. 2019. PMID: 30253328 Review.
Cited by
-
Magnetophoretic circuits: A review of device designs and implementation for precise single-cell manipulation.Anal Chim Acta. 2023 Sep 1;1272:341425. doi: 10.1016/j.aca.2023.341425. Epub 2023 May 31. Anal Chim Acta. 2023. PMID: 37355317 Free PMC article. Review.
-
Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels.Int J Mol Sci. 2023 Feb 8;24(4):3364. doi: 10.3390/ijms24043364. Int J Mol Sci. 2023. PMID: 36834776 Free PMC article.
-
Toward Single Cell Tattoos: Biotransfer Printing of Lithographic Gold Nanopatterns on Live Cells.Nano Lett. 2023 Aug 23;23(16):7477-7484. doi: 10.1021/acs.nanolett.3c01960. Epub 2023 Aug 1. Nano Lett. 2023. PMID: 37526201 Free PMC article.
-
Mechanical Forces Guide Axon Growth through the Nigrostriatal Pathway in an Organotypic Model.Adv Sci (Weinh). 2025 Aug;12(31):e2500400. doi: 10.1002/advs.202500400. Epub 2025 May 11. Adv Sci (Weinh). 2025. PMID: 40349175 Free PMC article.
-
Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation.Cancers (Basel). 2021 Sep 12;13(18):4583. doi: 10.3390/cancers13184583. Cancers (Basel). 2021. PMID: 34572810 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

