Calcium in Kenyon Cell Somata as a Substrate for an Olfactory Sensory Memory in Drosophila
- PMID: 29867361
- PMCID: PMC5960692
- DOI: 10.3389/fncel.2018.00128
Calcium in Kenyon Cell Somata as a Substrate for an Olfactory Sensory Memory in Drosophila
Erratum in
-
Corrigendum: Calcium in Kenyon Cell Somata as a Substrate for an Olfactory Sensory Memory in Drosophila.Front Cell Neurosci. 2018 Jul 3;12:197. doi: 10.3389/fncel.2018.00197. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30034325 Free PMC article.
Abstract
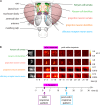
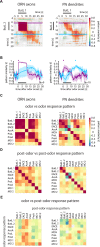
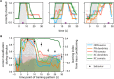
Animals can form associations between temporally separated stimuli. To do so, the nervous system has to retain a neural representation of the first stimulus until the second stimulus appears. The neural substrate of such sensory stimulus memories is unknown. Here, we search for a sensory odor memory in the insect olfactory system and characterize odorant-evoked Ca2+ activity at three consecutive layers of the olfactory system in Drosophila: in olfactory receptor neurons (ORNs) and projection neurons (PNs) in the antennal lobe, and in Kenyon cells (KCs) in the mushroom body. We show that the post-stimulus responses in ORN axons, PN dendrites, PN somata, and KC dendrites are odor-specific, but they are not predictive of the chemical identity of past olfactory stimuli. However, the post-stimulus responses in KC somata carry information about the identity of previous olfactory stimuli. These findings show that the Ca2+ dynamics in KC somata could encode a sensory memory of odorant identity and thus might serve as a basis for associations between temporally separated stimuli.
Keywords: Drosophila melanogaster; Kenyon cells; calcium imaging; mushroom body; olfaction; sensory memory; trace conditioning.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

