The Alteration of Emotion Regulation Precedes the Deficits in Interval Timing in the BACHD Rat Model for Huntington Disease
- PMID: 29867384
- PMCID: PMC5954136
- DOI: 10.3389/fnint.2018.00014
The Alteration of Emotion Regulation Precedes the Deficits in Interval Timing in the BACHD Rat Model for Huntington Disease
Abstract
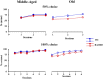
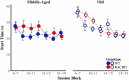
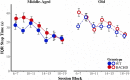
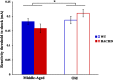
Huntington disease (HD) is an autosomal dominantly inherited, progressive neurodegenerative disorder which is accompanied by executive dysfunctions and emotional alteration. The aim of the present study was to assess the impact of emotion/stress on on-going highly demanding cognitive tasks, i.e., temporal processing, as a function of age in BACHD rats (a "full length" model of HD). Middle-aged (4-6 months) and old (10-12 months) rats were first trained on a 2 vs. 8-s temporal discrimination task, and then exposed to a series of bisection tests under normal and stressful (10 mild unpredictable foot-shocks) conditions. The animals were then trained on a peak interval task, in which reinforced fixed-interval (FI) 30-s trials were randomly intermixed with non-reinforced probe trials. After training, the effect of stress upon time perception was again assessed. Sensitivity to foot-shocks was also assessed independently. The results show effects of both age and genotype, with largely greater effects in old BACHD animals. The older BACHD animals had impaired learning in both tasks, but reached equivalent levels of performance as WT animals at the end of training in the temporal discrimination task, while remaining impaired in the peak interval task. Whereas sensitivity to foot-shock did not differ between BACHD and WT rats, delivery of foot-shocks during the test sessions had a disruptive impact on temporal behavior in WT animals, an effect which increased with age. In contrast, BACHD rats, independent of age, did not show any significant disruption under stress. In conclusion, BACHD rats showed a disruption in temporal learning in late symptomatic animals. Age-related modification in stress-induced impairment of temporal control of behavior was also observed, an effect which was greatly reduced in BACHD animals, thus confirming previous results suggesting reduced emotional reactivity in HD animals. The results suggest a staggered onset in cognitive and emotional alterations in HD, with emotional alteration being the earliest, possibly related to different time courses of degeneration in cortico-striatal and amygdala circuits.
Keywords: Huntington disease; interval timing; peak interval; stress; temporal bisection.
Figures







Similar articles
-
Altered reactivity of central amygdala to GABAAR antagonist in the BACHD rat model of Huntington disease.Neuropharmacology. 2017 Sep 1;123:136-147. doi: 10.1016/j.neuropharm.2017.05.032. Epub 2017 Jun 3. Neuropharmacology. 2017. PMID: 28587900
-
BACHD rats expressing full-length mutant huntingtin exhibit differences in social behavior compared to wild-type littermates.PLoS One. 2018 Feb 7;13(2):e0192289. doi: 10.1371/journal.pone.0192289. eCollection 2018. PLoS One. 2018. PMID: 29415038 Free PMC article.
-
Reduced impact of emotion on choice behavior in presymptomatic BACHD rats, a transgenic rodent model for Huntington Disease.Neurobiol Learn Mem. 2015 Nov;125:249-57. doi: 10.1016/j.nlm.2015.10.003. Epub 2015 Oct 14. Neurobiol Learn Mem. 2015. PMID: 26463506
-
Reversal learning and associative memory impairments in a BACHD rat model for Huntington disease.PLoS One. 2013 Nov 1;8(11):e71633. doi: 10.1371/journal.pone.0071633. eCollection 2013. PLoS One. 2013. PMID: 24223692 Free PMC article.
-
Cognitive deficits in bipolar disorders: Implications for emotion.Clin Psychol Rev. 2018 Feb;59:126-136. doi: 10.1016/j.cpr.2017.11.006. Epub 2017 Nov 21. Clin Psychol Rev. 2018. PMID: 29195773 Free PMC article. Review.
Cited by
-
The Peak Interval Procedure in Rodents: A Tool for Studying the Neurobiological Basis of Interval Timing and Its Alterations in Models of Human Disease.Bio Protoc. 2020 Sep 5;10(17):e3735. doi: 10.21769/BioProtoc.3735. eCollection 2020 Sep 5. Bio Protoc. 2020. PMID: 33659396 Free PMC article.
-
Respiration and brain neural dynamics associated with interval timing during odor fear learning in rats.Sci Rep. 2020 Oct 19;10(1):17643. doi: 10.1038/s41598-020-74741-2. Sci Rep. 2020. PMID: 33077831 Free PMC article.
-
Timing behavior in genetic murine models of neurological and psychiatric diseases.Exp Brain Res. 2021 Mar;239(3):699-717. doi: 10.1007/s00221-020-06021-4. Epub 2021 Jan 6. Exp Brain Res. 2021. PMID: 33404792 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

