Ginkgo biloba Extract Inhibits Astrocytic Lipocalin-2 Expression and Alleviates Neuroinflammatory Injury via the JAK2/STAT3 Pathway After Ischemic Brain Stroke
- PMID: 29867513
- PMCID: PMC5964562
- DOI: 10.3389/fphar.2018.00518
Ginkgo biloba Extract Inhibits Astrocytic Lipocalin-2 Expression and Alleviates Neuroinflammatory Injury via the JAK2/STAT3 Pathway After Ischemic Brain Stroke
Abstract
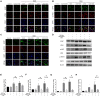
Background: Astrogliosis has the potential to lead to harmful effects, namely, neuroinflammation, and to interfere with synapse sprouting. Previous studies have suggested that Lipocalin-2 (LCN2) acts as a key target in regulating the reaction of astrocytes. However, the underlying molecular mechanism is not fully elucidated. In the present study, we examined the neuroprotective and anti-inflammatory effects of Ginkgo biloba extract (EGB), a well-known extract with potential immunoregulatory properties in the nervous system. Methods: Triphenyltetrazolium chloride staining, hematoxylin-eosin staining, electron microscopy, and neurological assessments were performed in a microsphere-embolized rat model. Human astrocytes exposed to oxygen glucose deprivation (OGD) were used for in vitro experiments. Inflammatory cytokines, multi-labeling immunofluorescence, and Western blotting were used to investigate the molecular mechanisms underlying the EGB-mediated anti-inflammatory effects in vivo and in vitro. Results: EGB markedly attenuated cerebral infarction and neuronal apoptosis, reduced the inflammatory cytokine level, and alleviated neurological deficiencies in cerebral ischemic rats. After surgery, EGB significantly inhibited astrocyte activation, reduced the phosphorylation of STAT3 and JAK2 and decreased LCN2 expression. In vitro, EGB blocked OGD-induced STAT3 activation and the generation of pro-inflammatory cytokines in human astrocytes, and these effects were significantly enhanced by LCN2 overexpression. EGB downregulated these effects enhanced by LCN2 overexpression. Conclusion: EGB is demonstrated to mediate neuroinflammation, which protects against ischemic brain injury by inhibiting astrogliosis and suppresses neuroinflammation via the LCN2-JAK2/STAT3 pathway, providing insight into a promising therapeutic strategy for ischemic stroke.
Keywords: Ginkgo biloba extract; Lipocalin-2 (LCN2); astrocyte; cerebral ischemia; neuroinflammation.
Figures




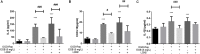
References
-
- Baliutyte G., Trumbeckaite S., Baniene R., Borutaite V., Toleikis A. (2014). Effects of standardized extract of Ginkgo biloba leaves EGb761 on mitochondrial functions: mechanism(s) of action and dependence on the source of mitochondria and respiratory substrate. 46 493–501. 10.1007/s10863-014-9590-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

