Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits
- PMID: 29867586
- PMCID: PMC5968099
- DOI: 10.3389/fphys.2018.00565
Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits
Abstract
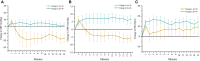
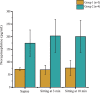
Disruption of motor and autonomic pathways induced by spinal cord injury (SCI) often leads to persistent low arterial blood pressure and orthostatic intolerance. Spinal cord epidural stimulation (scES) has been shown to enable independent standing and voluntary movement in individuals with clinically motor complete SCI. In this study, we addressed whether scES configured to activate motor lumbosacral networks can also modulate arterial blood pressure by assessing continuous, beat-by-beat blood pressure and lower extremity electromyography during supine and standing in seven individuals with C5-T4 SCI. In three research participants with arterial hypotension, orthostatic intolerance, and low levels of circulating catecholamines (group 1), scES applied while supine and standing resulted in increased arterial blood pressure. In four research participants without evidence of arterial hypotension or orthostatic intolerance and normative circulating catecholamines (group 2), scES did not induce significant increases in arterial blood pressure. During scES, there were no significant differences in electromyographic (EMG) activity between group 1 and group 2. In group 1, during standing assisted by scES, blood pressure was maintained at 119/72 ± 7/14 mmHg (mean ± SD) compared with 70/45 ± 5/7 mmHg without scES. In group 2 there were no arterial blood pressure changes during standing with or without scES. These findings demonstrate that scES configured to facilitate motor function can acutely increase arterial blood pressure in individuals with SCI-induced cardiovascular deficits.
Keywords: blood pressure; epidural stimulation; human spinal cord injury; orthostatic hypotension; systemic hypotension.
Figures




References
-
- Carlozzi N. E., Fyffe D., Morin K. G., Byrne R., Tulsky D. S., Victorson D., et al. (2013). Impact of blood pressure dysregulation on health-related quality of life in persons with spinal cord injury: development of a conceptual model. Arch. Phys. Med. Rehabil. 94, 1721–1730. 10.1016/j.apmr.2013.02.024 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

