In Vivo and In Vitro Impact of Carbohydrate Variation on Human Follicle-Stimulating Hormone Function
- PMID: 29867757
- PMCID: PMC5960776
- DOI: 10.3389/fendo.2018.00216
In Vivo and In Vitro Impact of Carbohydrate Variation on Human Follicle-Stimulating Hormone Function
Abstract
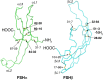
Human follicle-stimulating hormone (FSH) exhibits both macro- and microheterogeneity in its carbohydrate moieties. Macroheterogeneity results in three physiologically relevant FSHβ subunit variants, two that possess a single N-linked glycan at either one of the two βL1 loop glycosylation sites or one with both glycans. Microheterogeneity is characterized by 80 to over 100 unique oligosaccharide structures attached to each of the 3 to 4 occupied N-glycosylation sites. With respect to its receptor, partially glycosylated (hypo-glycosylated) FSH variants exhibit higher association rates, greater apparent affinity, and greater occupancy than fully glycosylated FSH. Higher receptor binding-activity is reflected by greater in vitro bioactivity and, in some cases, greater in vivo bioactivity. Partially glycosylated pituitary FSH shows an age-related decline in abundance that may be associated with decreased fertility. In this review, we describe an integrated approach involving genetic models, in vitro signaling studies, FSH biochemistry, relevance of physiological changes in FSH glycoform abundance, and characterize the impact of FSH macroheterogeneity on fertility and reproductive aging. We will also address the controversy with regard to claims of a direct action of FSH in mediating bone loss especially at the peri- and postmenopausal stages.
Keywords: N-glycosylation; bone; female Infertility; follicle-stimulating hormone; pituitary.
Figures


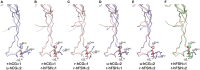

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

