Brain Aromatase Modulates Serotonergic Neuron by Regulating Serotonin Levels in Zebrafish Embryos and Larvae
- PMID: 29867763
- PMCID: PMC5954033
- DOI: 10.3389/fendo.2018.00230
Brain Aromatase Modulates Serotonergic Neuron by Regulating Serotonin Levels in Zebrafish Embryos and Larvae
Abstract
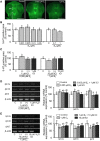
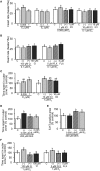
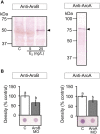
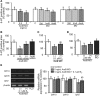
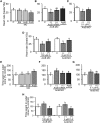
Teleost fish are known to express two isoforms of P450 aromatase, a key enzyme for estrogen synthesis. One of the isoforms, brain aromatase (AroB), cyp19a1b, is highly expressed during early development of zebrafish, thereby suggesting its role in brain development. On the other hand, early development of serotonergic neuron, one of the major monoamine neurons, is considered to play an important role in neurogenesis. Therefore, in this study, we investigated the role of AroB in development of serotonergic neuron by testing the effects of (1) estradiol (E2) exposure and (2) morpholino (MO)-mediated AroB knockdown. When embryos were exposed to E2, the effects were biphasic. The low dose of E2 (0.005 µM) significantly increased serotonin (5-HT) positive area at 48 hour post-fertilization (hpf) detected by immunohistochemistry and relative mRNA levels of tryptophan hydroxylase isoforms (tph1a, tph1b, and tph2) at 96 hpf measured by semi-quantitative PCR. To test the effects on serotonin transmission, heart rate and thigmotaxis, an indicator of anxiety, were analyzed. The low dose also significantly increased heart rate at 48 hpf and decreased thigmotaxis. The high dose of E2 (1 µM) exhibited opposite effects in all parameters. The effects of both low and high doses were reversed by addition of estrogen receptor (ER) blocker, ICI 182,780, thereby suggesting that the effects were mediated through ER. When AroB MO was injected to fertilized eggs, 5-HT-positive area was significantly decreased, while the significant decrease in relative tph mRNA levels was found only with tph2 but not with two other isoforms. AroB MO also decreased heart rate and increased thigmotaxis. All the effects were rescued by co-injection with AroB mRNA and by exposure to E2. Taken together, this study demonstrates the role of brain aromatase in development of serotonergic neuron in zebrafish embryos and larvae, implying that brain-formed estrogen is an important factor to sustain early development of serotonergic neuron.
Keywords: biphasic manner; brain aromatase; early development; estradiol; serotonergic neuron; zebrafish.
Figures
References
-
- Callard GV, Greytak SR, Novillo A, Cotter CA, Mayer RK. Brain aromatase in fish: perspective and comparative approaches. In: Balthazart J, Ball G, editors. Brain Aromatase, Estrogen, and Behavior. New York: Oxford University Press; (2013). p. 13–42.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

