New Insights on Steroid Biotechnology
- PMID: 29867863
- PMCID: PMC5962712
- DOI: 10.3389/fmicb.2018.00958
New Insights on Steroid Biotechnology
Abstract
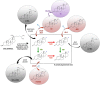
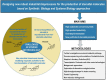
Nowadays steroid manufacturing occupies a prominent place in the pharmaceutical industry with an annual global market over $10 billion. The synthesis of steroidal active pharmaceutical ingredients (APIs) such as sex hormones (estrogens, androgens, and progestogens) and corticosteroids is currently performed by a combination of microbiological and chemical processes. Several mycobacterial strains capable of naturally metabolizing sterols (e.g., cholesterol, phytosterols) are used as biocatalysts to transform phytosterols into steroidal intermediates (synthons), which are subsequently used as key precursors to produce steroidal APIs in chemical processes. These synthons can also be modified by other microbial strains capable of introducing regio- and/or stereospecific modifications (functionalization) into steroidal molecules. Most of the industrial microbial strains currently available have been improved through traditional technologies based on physicochemical mutagenesis and selection processes. Surprisingly, Synthetic Biology and Systems Biology approaches have hardly been applied for this purpose. This review attempts to highlight the most relevant research on Steroid Biotechnology carried out in last decades, focusing specially on those works based on recombinant DNA technologies, as well as outlining trends and future perspectives. In addition, the need to construct new microbial cell factories (MCF) to design more robust and bio-sustainable bioprocesses with the ultimate aim of producing steroids à la carte is discussed.
Keywords: actinobacteria; metabolic engineering; microbial cell factory; pharmaceuticals; steroid; systems biology.
Figures



References
-
- Andor A., Jekkel A., Hopwood D. A., Jeanplong F., Ilkoy E., Kónya A., et al. . (2006). Generation of useful insertionally blocked sterol degradation pathway mutants of fast-growing mycobacteria and cloning, characterization, and expression of the terminal oxygenase of the 3- ketosteroid 9alpha-hydroxylase in Mycobacterium smegmatis mc(2)155. Appl. Environ. Microbiol. 72, 6554–6559. 10.1128/AEM.00941-06 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

