Scavenging Circulating Mitochondrial DNA as a Potential Therapeutic Option for Multiple Organ Dysfunction in Trauma Hemorrhage
- PMID: 29867926
- PMCID: PMC5951958
- DOI: 10.3389/fimmu.2018.00891
Scavenging Circulating Mitochondrial DNA as a Potential Therapeutic Option for Multiple Organ Dysfunction in Trauma Hemorrhage
Abstract
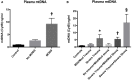
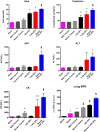
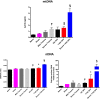
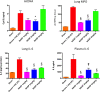
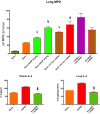
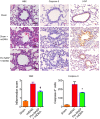
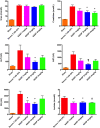
Trauma is a leading cause of death worldwide with 5.8 million deaths occurring yearly. Almost 40% of trauma deaths are due to bleeding and occur in the first few hours after injury. Of the remaining severely injured patients up to 25% develop a dysregulated immune response leading to multiple organ dysfunction syndrome (MODS). Despite improvements in trauma care, the morbidity and mortality of this condition remains very high. Massive traumatic injury can overwhelm endogenous homeostatic mechanisms even with prompt treatment. The underlying mechanisms driving MODS are also not fully elucidated. As a result, successful therapies for trauma-related MODS are lacking. Trauma causes tissue damage that releases a large number of endogenous damage-associated molecular patterns (DAMPs). Mitochondrial DAMPs released in trauma, such as mitochondrial DNA (mtDNA), could help to explain part of the immune response in trauma given the structural similarities between mitochondria and bacteria. MtDNA, like bacterial DNA, contains an abundance of highly stimulatory unmethylated CpG DNA motifs that signal through toll-like receptor-9 to produce inflammation. MtDNA has been shown to be highly damaging when injected into healthy animals causing acute organ injury to develop. Elevated circulating levels of mtDNA have been reported in trauma patients but an association with clinically meaningful outcomes has not been established in a large cohort. We aimed to determine whether mtDNA released after clinical trauma hemorrhage is sufficient for the development of MODS. Secondly, we aimed to determine the extent of mtDNA release with varying degrees of tissue injury and hemorrhagic shock in a clinically relevant rodent model. Our final aim was to determine whether neutralizing mtDNA with the nucleic acid scavenging polymer, hexadimethrine bromide (HDMBr), at a clinically relevant time point in vivo would reduce the severity of organ injury in this model.
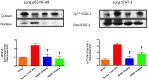
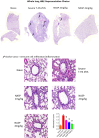
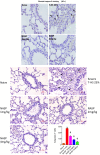
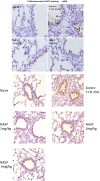
Conclusions: We have shown that the release of mtDNA is sufficient for the development of multiple organ injury. MtDNA concentrations likely peak at different points in the early postinjury phase dependent on the degree of isolated trauma vs combined trauma and hemorrhagic shock. HDMBr scavenging of circulating mtDNA (and nuclear DNA, nDNA) is associated with rescue from severe multiple organ injury in the animal model. This suggests that HDMBr could have utility in rescue from human trauma-induced MODS.
Keywords: Toll-like receptor-9; damage-associated molecular patterns; mitochondrial DNA; multiple organ dysfunction syndrome; nucleic acid scavenger; sterile inflammation; trauma; trauma hemorrhage.
Figures

References
-
- WHO. Global Health Estimates 2015 Summary Tables: Deaths by Cause, Age and Sex, 2000–2015 [Online]. World Health Organization; (2016). Available from:http://www.who.int/healthinfo/global_burden_disease?GHE2015_Deaths_Globa... (Accessed: October 01, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

