Interleukin-1α Mediates Ozone-Induced Myeloid Differentiation Factor-88-Dependent Epithelial Tissue Injury and Inflammation
- PMID: 29867931
- PMCID: PMC5950844
- DOI: 10.3389/fimmu.2018.00916
Interleukin-1α Mediates Ozone-Induced Myeloid Differentiation Factor-88-Dependent Epithelial Tissue Injury and Inflammation
Abstract
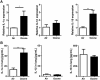
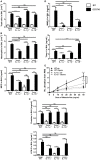
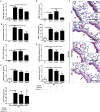
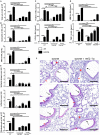
Air pollution associated with ozone exposure represents a major inducer of respiratory disease in man. In mice, a single ozone exposure causes lung injury with disruption of the respiratory barrier and inflammation. We investigated the role of interleukin-1 (IL-1)-associated cytokines upon a single ozone exposure (1 ppm for 1 h) using IL-1α-, IL-1β-, and IL-18-deficient mice or an anti-IL-1α neutralizing antibody underlying the rapid epithelial cell death. Here, we demonstrate the release of the alarmin IL-1α after ozone exposure and that the acute respiratory barrier injury and inflammation and airway hyperreactivity are IL-1α-dependent. IL-1α signaling via IL-1R1 depends on the adaptor protein myeloid differentiation factor-88 (MyD88). Importantly, epithelial cell signaling is critical, since deletion of MyD88 in lung type I alveolar epithelial cells reduced ozone-induced inflammation. In addition, intratracheal injection of recombinant rmIL-1α in MyD88acid mice led to reduction of inflammation in comparison with wild type mice treated with rmIL-1α. Therefore, a major part of inflammation is mediated by IL-1α signaling in epithelial cells. In conclusion, the alarmin IL-1α released upon ozone-induced tissue damage and inflammation is mediated by MyD88 signaling in epithelial cells. Therefore, IL-1α may represent a therapeutic target to attenuate ozone-induced lung inflammation and hyperreactivity.
Keywords: epithelial cell; inflammation; interleukin-18; interleukin-1α; interleukin-1β; myeloid differentiation factor-88; ozone.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

