"Immune TOR-opathies," a Novel Disease Entity in Clinical Immunology
- PMID: 29867948
- PMCID: PMC5954032
- DOI: 10.3389/fimmu.2018.00966
"Immune TOR-opathies," a Novel Disease Entity in Clinical Immunology
Abstract
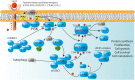
Primary immunodeficiencies (PIDs) represent a group of mostly monogenic disorders caused by loss- or gain-of-function mutations in over 340 known genes that lead to abnormalities in the development and/or the function of the immune system. However, mutations in different genes can affect the same cell-signaling pathway and result in overlapping clinical phenotypes. In particular, mutations in the genes encoding for members of the phosphoinositide3-kinase (PI3K)/AKT/mTOR/S6 kinase (S6K) signaling cascade or for molecules interacting with this pathway have been associated with different PIDs that are often characterized by the coexistence of both immune deficiency and autoimmunity. The serine/threonine kinase mechanistic/mammalian target of rapamycin (mTOR), which acts downstream of PI3K and AKT, is emerging as a key regulator of immune responses. It integrates a variety of signals from the microenvironment to control cell growth, proliferation, and metabolism. mTOR plays therefore a central role in the regulation of immune cells' differentiation and functions. Here, we review the different PIDs that share an impairment of the PI3K/AKT/mTOR/S6K pathway and we propose to name them "immune TOR-opathies" by analogy with a group of neurological disorders that has been originally defined by PB Crino and that are due to aberrant mTOR signaling (1). A better understanding of the role played by this complex intracellular cascade in the pathophysiology of "immune TOR-opathies" is crucial to develop targeted therapies.
Keywords: AKT; PI3k; S6K; immune dysregulation; kinase; mTOR; primary immunodeficiency.
Figures
References
-
- Crino PB. Focal brain malformations: a spectrum of disorders along the mTOR cascade. Novartis Found Symp (2007) 288:260–72; discussion 272–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

