NoxO1 Controls Proliferation of Colon Epithelial Cells
- PMID: 29867954
- PMCID: PMC5951971
- DOI: 10.3389/fimmu.2018.00973
NoxO1 Controls Proliferation of Colon Epithelial Cells
Abstract
Aim: Reactive oxygen species (ROS) produced by enzymes of the NADPH oxidase family serve as second messengers for cellular signaling. Processes such as differentiation and proliferation are regulated by NADPH oxidases. In the intestine, due to the exceedingly fast and constant renewal of the epithelium both processes have to be highly controlled and balanced. Nox1 is the major NADPH oxidase expressed in the gut, and its function is regulated by cytosolic subunits such as NoxO1. We hypothesize that the NoxO1-controlled activity of Nox1 contributes to a proper epithelial homeostasis and renewal in the gut.
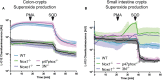
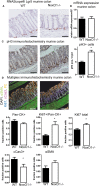
Results: NoxO1 is highly expressed in the colon. Knockout of NoxO1 reduces the production of superoxide in colon crypts and is not subsidized by an elevated expression of its homolog p47phox. Knockout of NoxO1 increases the proliferative capacity and prevents apoptosis of colon epithelial cells. In mouse models of dextran sulfate sodium (DSS)-induced colitis and azoxymethane/DSS induced colon cancer, NoxO1 has a protective role and may influence the population of natural killer cells.
Conclusion: NoxO1 affects colon epithelium homeostasis and prevents inflammation.
Keywords: Nox1; NoxO1; colon; inflammation; proliferation; reactive oxygen species.
Figures



References
-
- Di Marco E, Gray SP, Kennedy K, Szyndralewiez C, Lyle AN, Lassègue B, et al. NOX4-derived reactive oxygen species limit fibrosis and inhibit proliferation of vascular smooth muscle cells in diabetic atherosclerosis. Free Radic Biol Med (2016) 97:556–67.10.1016/j.freeradbiomed.2016.07.013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

