Meta-Analysis of Maternal and Fetal Transcriptomic Data Elucidates the Role of Adaptive and Innate Immunity in Preterm Birth
- PMID: 29867970
- PMCID: PMC5954243
- DOI: 10.3389/fimmu.2018.00993
Meta-Analysis of Maternal and Fetal Transcriptomic Data Elucidates the Role of Adaptive and Innate Immunity in Preterm Birth
Abstract
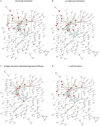
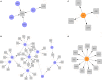
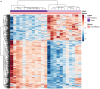
Preterm birth (PTB) is the leading cause of newborn deaths around the world. Spontaneous preterm birth (sPTB) accounts for two-thirds of all PTBs; however, there remains an unmet need of detecting and preventing sPTB. Although the dysregulation of the immune system has been implicated in various studies, small sizes and irreproducibility of results have limited identification of its role. Here, we present a cross-study meta-analysis to evaluate genome-wide differential gene expression signals in sPTB. A comprehensive search of the NIH genomic database for studies related to sPTB with maternal whole blood samples resulted in data from three separate studies consisting of 339 samples. After aggregating and normalizing these transcriptomic datasets and performing a meta-analysis, we identified 210 genes that were differentially expressed in sPTB relative to term birth. These genes were enriched in immune-related pathways, showing upregulation of innate immunity and downregulation of adaptive immunity in women who delivered preterm. An additional analysis found several of these differentially expressed at mid-gestation, suggesting their potential to be clinically relevant biomarkers. Furthermore, a complementary analysis identified 473 genes differentially expressed in preterm cord blood samples. However, these genes demonstrated downregulation of the innate immune system, a stark contrast to findings using maternal blood samples. These immune-related findings were further confirmed by cell deconvolution as well as upstream transcription and cytokine regulation analyses. Overall, this study identified a strong immune signature related to sPTB as well as several potential biomarkers that could be translated to clinical use.
Keywords: immunology; meta-analysis; pregnancy; preterm birth; transcriptomics.
Figures






References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (2012) 379:2162–72. 10.1016/S0140-6736(12)60820-4 - DOI - PubMed

