Distribution and Interaction of Murine Pulmonary Phagocytes in the Naive and Allergic Lung
- PMID: 29868009
- PMCID: PMC5964136
- DOI: 10.3389/fimmu.2018.01046
Distribution and Interaction of Murine Pulmonary Phagocytes in the Naive and Allergic Lung
Abstract
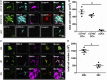
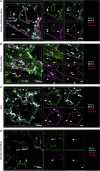
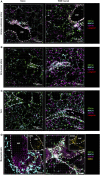
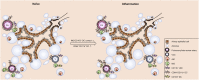
The division of labor between pulmonary phagocytic subsets [macrophage/monocyte and dendritic cell (DC) subpopulations] has been described at the functional level. However, whether these lung phagocytes also display unique spatial distribution remains unclear. Here, to analyze cellular distribution in lung compartments and contacts between phagocyte subpopulations, we established an immunohistochemistry (IHC)-based method to clearly identify murine lung phagocyte subsets in situ based on differential expression of CD11c, CD11b, MHC-II, Langerin and mPDCA-1. Furthermore, we investigated subset-specific functional differences in antigen uptake and spatial changes upon allergic sensitization. Our staining allowed the distinction between alveolar macrophages (AMs), interstitial macrophage (IM) subpopulations, CD11b+ DC subpopulations, CD103+ DCs, and plasmacytoid DCs (pDCs). We identified interstitial regions between airways and around airways as regions of IM/CD11b+ DC/CD103+ DC clusters, where a subset of IMs (IM2) and CD103+ DCs formed intense contacts that decreased upon allergic sensitization. These data indicate functional interactions between both cell types either in steady state or after antigen encounter affecting the development of allergies or tolerance. Furthermore, we observed major antigen uptake in AMs and IMs rather than DC subpopulations that was not restricted to airways and adjacent areas. This will enable to focus future studies to immunologically relevant cellular interactions and to unravel which cells are tipping the balance between pro-inflammatory immune responses or tolerance.
Keywords: allergic airway disease; antigen uptake; dendritic cells; immunohistochemistry; lung; macrophages; spatial distribution.
Figures





Similar articles
-
Transcriptional Changes in Pulmonary Phagocyte Subsets Dictate the Outcome Following Interaction With The Fungal Pathogen Cryptococcus neoformans.Front Immunol. 2021 Sep 28;12:722500. doi: 10.3389/fimmu.2021.722500. eCollection 2021. Front Immunol. 2021. PMID: 34650554 Free PMC article.
-
A Consistent Method to Identify and Isolate Mononuclear Phagocytes from Human Lung and Lymph Nodes.Methods Mol Biol. 2018;1799:381-395. doi: 10.1007/978-1-4939-7896-0_28. Methods Mol Biol. 2018. PMID: 29956166 Free PMC article.
-
Aeroallergen challenge promotes dendritic cell proliferation in the airways.J Immunol. 2013 Feb 1;190(3):897-903. doi: 10.4049/jimmunol.1200220. Epub 2012 Dec 24. J Immunol. 2013. PMID: 23267021
-
Origin, Localization, and Immunoregulatory Properties of Pulmonary Phagocytes in Allergic Asthma.Front Immunol. 2016 Mar 23;7:107. doi: 10.3389/fimmu.2016.00107. eCollection 2016. Front Immunol. 2016. PMID: 27047494 Free PMC article. Review.
-
Airway Macrophage and Dendritic Cell Subsets in the Resting Human Lung.Crit Rev Immunol. 2018;38(4):303-331. doi: 10.1615/CritRevImmunol.2018026459. Crit Rev Immunol. 2018. PMID: 30806245 Free PMC article. Review.
Cited by
-
Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice.J Fungi (Basel). 2022 Jul 28;8(8):792. doi: 10.3390/jof8080792. J Fungi (Basel). 2022. PMID: 36012781 Free PMC article.
-
Too young to die? How aging affects cellular innate immune responses to influenza virus and disease severity.Virulence. 2021 Dec;12(1):1629-1646. doi: 10.1080/21505594.2021.1939608. Virulence. 2021. PMID: 34152253 Free PMC article. Review.
-
The impact of the lung environment on macrophage development, activation and function: diversity in the face of adversity.Mucosal Immunol. 2022 Feb;15(2):223-234. doi: 10.1038/s41385-021-00480-w. Epub 2022 Jan 11. Mucosal Immunol. 2022. PMID: 35017701 Free PMC article. Review.
-
Transcriptional Changes in Pulmonary Phagocyte Subsets Dictate the Outcome Following Interaction With The Fungal Pathogen Cryptococcus neoformans.Front Immunol. 2021 Sep 28;12:722500. doi: 10.3389/fimmu.2021.722500. eCollection 2021. Front Immunol. 2021. PMID: 34650554 Free PMC article.
-
Pulmonary Macrophage and Dendritic Cell Responses to Cryptococcus neoformans.Front Cell Infect Microbiol. 2020 Feb 11;10:37. doi: 10.3389/fcimb.2020.00037. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32117810 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

