Protective Role of Myeloid Cells Expressing a G-CSF Receptor Polymorphism in an Induced Model of Lupus
- PMID: 29868014
- PMCID: PMC5954343
- DOI: 10.3389/fimmu.2018.01053
Protective Role of Myeloid Cells Expressing a G-CSF Receptor Polymorphism in an Induced Model of Lupus
Abstract
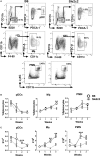
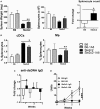
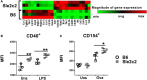
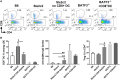
The genetic analysis of the lupus-prone NZM2410 mouse has identified a suppressor locus, Sle2c2, which confers resistance to spontaneous lupus in combination with NZM2410 susceptibility loci, or in the chronic graft-versus-host disease (cGVHD) induced model of lupus in the B6.Sle2c2 congenic strain. The candidate gene for Sle2c2, the Csf3r gene encoding the granulocyte colony-stimulating factor receptor (G-CSF-R/CD114), was validated when cGVHD was restored in B6.Sle2c2 mice after treatment with G-CSF. The goal of the project reported herein was to investigate the myeloid cells that confer resistance to cGVHD and to ascertain if the mechanism behind their suppression involves the G-CSF pathway. We showed that despite expressing the highest levels of G-CSF-R, neutrophils play only a modest role in the autoimmune activation induced by cGVHD. We also found reduced expression levels of G-CSF-R on the surface of dendritic cells (DCs) and a differential distribution of DC subsets in response to cGVHD in B6.Sle2c2 versus B6 mice. The CD8α+ DC subset, known for its tolerogenic phenotype, was expanded upon induction of cGVHD in B6.Sle2c2 mice. In addition, the deficiency of CD8α+ DC subset enhanced the severity of cGVHD in B6.Batf3-/- and B6.Sle2c2 mice, confirming their role in suppression of cGVHD. B6.Sle2c2DCs presented lowered activation and antigen presentation abilities and expressed lower levels of genes associated with DC activation and maturation. Exposure to exogenous G-CSF reversed the majority of these phenotypes, suggesting that tolerogenic DCs maintained through a defective G-CSF-R pathway mediated the resistance to cGVHD in B6.Sle2c2 mice.
Keywords: G-CSF-R; lupus; neutrophils; suppressive allele; tolerogenic dendritic cells.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

