Fluorescent Tracking of Yeast Division Clarifies the Essential Role of Spleen Tyrosine Kinase in the Intracellular Control of Candida glabrata in Macrophages
- PMID: 29868018
- PMCID: PMC5964189
- DOI: 10.3389/fimmu.2018.01058
Fluorescent Tracking of Yeast Division Clarifies the Essential Role of Spleen Tyrosine Kinase in the Intracellular Control of Candida glabrata in Macrophages
Abstract
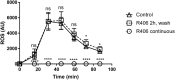
Macrophages play a critical role in the elimination of fungal pathogens. They are sensed via cell surface pattern-recognition receptors and are phagocytosed into newly formed organelles called phagosomes. Phagosomes mature through the recruitment of proteins and lysosomes, resulting in addition of proteolytic enzymes and acidification of the microenvironment. Our earlier studies demonstrated an essential role of Dectin-1-dependent activation of spleen tyrosine kinase (Syk) in the maturation of fungal containing phagosomes. The absence of Syk activity interrupted phago-lysosomal fusion resulting in arrest at an early phagosome stage. In this study, we sought to define the contribution of Syk to the control of phagocytosed live Candida glabrata in primary macrophages. To accurately measure intracellular yeast division, we designed a carboxyfluorescein succinimidyl ester (CFSE) yeast division assay in which bright fluorescent parent cells give rise to dim daughter cells. The CFSE-labeling of C. glabrata did not affect the growth rate of the yeast. Following incubation with macrophages, internalized CFSE-labeled C. glabrata were retrieved by cellular lysis, tagged using ConA-647, and the amount of residual CFSE fluorescence was assessed by flow cytometry. C. glabrata remained undivided (CFSE bright) for up to 18 h in co-culture with primary macrophages. Treatment of macrophages with R406, a specific Syk inhibitor, resulted in loss of intracellular control of C. glabrata with initiation of division within 4 h. Delayed Syk inhibition after 8 h was less effective indicating that Syk is critically required at early stages of macrophage-fungal interaction. In conclusion, we demonstrate a new method of tracking division of C. glabrata using CFSE labeling. Our results suggest that early Syk activation is essential for macrophage control of phagocytosed C. glabrata.
Keywords: Candida; Candida glabrata; carboxyfluorescein succinimidyl ester; macrophages; phagosome; spleen tyrosine kinase.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

