FcpB Is a Surface Filament Protein of the Endoflagellum Required for the Motility of the Spirochete Leptospira
- PMID: 29868490
- PMCID: PMC5953323
- DOI: 10.3389/fcimb.2018.00130
FcpB Is a Surface Filament Protein of the Endoflagellum Required for the Motility of the Spirochete Leptospira
Abstract
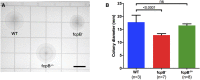
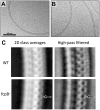
The spirochete endoflagellum is a unique motility apparatus among bacteria. Despite its critical importance for pathogenesis, the full composition of the flagellum remains to be determined. We have recently reported that FcpA is a novel flagellar protein and a major component of the sheath of the filament of the spirochete Leptospira. By screening a library of random transposon mutants in the spirochete Leptospira biflexa, we found a motility-deficient mutant harboring a disruption in a hypothetical gene of unknown function. Here, we show that this gene encodes a surface component of the endoflagellar filament and is required for typical hook- and spiral-shaped ends of the cell body, coiled structure of the endoflagella, and high velocity phenotype. We therefore named the gene fcpB for flagellar-coiling protein B. fcpB is conserved in all members of the Leptospira genus, but not present in other organisms including other spirochetes. Complementation of the fcpB- mutant restored the wild-type morphology and motility phenotypes. Immunoblotting with anti-FcpA and anti-FcpB antisera and cryo-electron microscopy of the filament indicated that FcpB assembled onto the surface of the sheath of the filament and mostly located on the outer (convex) side of the coiled filament. We provide evidence that FcpB, together with FcpA, are Leptospira-specific novel components of the sheath of the filament, key determinants of the coiled and asymmetric structure of the endoflagella and are essential for high velocity. Defining the components of the endoflagella and their functions in these atypical bacteria should greatly enhance our understanding of the mechanisms by which these bacteria produce motility.
Keywords: Leptospira; flagella; flagellar system; motility; spirochetes.
Figures




References
-
- Fontana C., Lambert A., Benaroudj N., Gasparini D., Gorgette O., Cachet N., et al. . (2016). Analysis of a spontaneous non-motile and avirulent mutant shows that FliM is Required for full endoflagella assembly in Leptospira interrogans. PLoS ONE 11:e0152916. 10.1371/journal.pone.0152916 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

