M Protein of Group a Streptococcus Plays an Essential Role in Inducing High Expression of A20 in Macrophages Resulting in the Downregulation of Inflammatory Response in Lung Tissue
- PMID: 29868491
- PMCID: PMC5968387
- DOI: 10.3389/fcimb.2018.00131
M Protein of Group a Streptococcus Plays an Essential Role in Inducing High Expression of A20 in Macrophages Resulting in the Downregulation of Inflammatory Response in Lung Tissue
Abstract
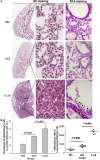
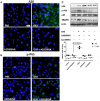
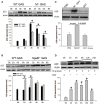
Group A streptococcus (GAS), a common pathogen, is able to escape host immune attack and thus survive for longer periods of time. One of the mechanisms used by GAS is the upregulated expression of immunosuppressive molecules, which leads to a reduction in the production of inflammatory cytokines in immune cells. In the present study, we found that macrophages produced lower levels of proinflammatory cytokines (IL-1β, TNF-α, IL-6) when challenged with GAS than they did when challenged with Escherichia coli (E. coli). Simultaneously, in a mouse model of lung infection, GAS appeared to induce a weaker inflammatory response compared to E. coli. Our data also indicated that the expression of the A20 transcriptional regulator was higher in GAS-infected macrophages than that in macrophages infected with E. coli, and that high expression of A20 correlated with a reduction in the production of TRAF6. SiRNA targeting of A20 led to the increased production of TRAF6, IL-1β, TNF-α, and IL-6, suggesting that A20 inhibits synthesis of these key proinflammatory cytokines. We also investigated the pathway underlying A20 production and found that the synthesis of A20 depends on My88, and to a lower extent on TNFR1. Finally, we showed a significant reduction in the expression of A20 in macrophages stimulated by M protein-mutant GAS, however, a speB-GAS mutant, which is unable to degrade M protein, induced a greater level of A20 production than wild type GAS. Collectively, our data suggested that M protein of GAS was responsible for inducing A20 expression in macrophages, which in turn down-regulates the inflammatory cytokine response in order to facilitate GAS in evading immune surveillance and thus prolong survival in the host.
Keywords: A20; Group A Streptococcus (GAS); M protein; macrophages; negative regulation.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

