The in Vitro Antigenicity of Plasmodium vivax Rhoptry Neck Protein 2 (Pv RON2) B- and T-Epitopes Selected by HLA-DRB1 Binding Profile
- PMID: 29868512
- PMCID: PMC5962679
- DOI: 10.3389/fcimb.2018.00156
The in Vitro Antigenicity of Plasmodium vivax Rhoptry Neck Protein 2 (Pv RON2) B- and T-Epitopes Selected by HLA-DRB1 Binding Profile
Abstract
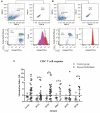
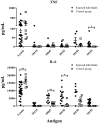
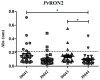
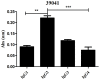
Malaria caused by Plasmodium vivax is a neglected disease which is responsible for the highest morbidity in both Americas and Asia. Despite continuous public health efforts to prevent malarial infection, an effective antimalarial vaccine is still urgently needed. P. vivax vaccine development involves analyzing naturally-infected patients' immune response to the specific proteins involved in red blood cell invasion. The P. vivax rhoptry neck protein 2 (PvRON2) is a highly conserved protein which is expressed in late schizont rhoptries; it interacts directly with AMA-1 and might be involved in moving-junction formation. Bioinformatics approaches were used here to select B- and T-cell epitopes. Eleven high-affinity binding peptides were selected using the NetMHCIIpan-3.0 in silico prediction tool; their in vitro binding to HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*1101 or HLA-DRB1*1302 was experimentally assessed. Four peptides (39152 (HLA-DRB1*04 and 11), 39047 (HLA-DRB1*07), 39154 (HLADRB1*13) and universal peptide 39153) evoked a naturally-acquired T-cell immune response in P. vivax-exposed individuals from two endemic areas in Colombia. All four peptides had an SI greater than 2 in proliferation assays; however, only peptides 39154 and 39153 had significant differences compared to the control group. Peptide 39047 was able to significantly stimulate TNF and IL-10 production while 39154 stimulated TNF production. Allele-specific peptides (but not the universal one) were able to stimulate IL-6 production; however, none induced IFN-γ production. The Bepipred 1.0 tool was used for selecting four B-cell epitopes in silico regarding humoral response. Peptide 39041 was the only one recognized by P. vivax-exposed individuals' sera and had significant differences concerning IgG subclasses; an IgG2 > IgG4 profile was observed for this peptide, agreeing with a protection-inducing role against P. falciparum and P. vivax as previously described for antigens such as RESA and MSP2. The bioinformatics results and in vitro evaluation reported here highlighted two T-cell epitopes (39047 and 39154) being recognized by memory cells and a B-cell epitope (39041) identified by P. vivax-exposed individuals' sera which could be used as potential candidates when designing a subunit-based vaccine.
Keywords: HLA-DRB1 typing; Plasmodium vivax; PvRON2; antigenicity; cellular and humoral response; epitope; synthetic peptide.
Figures




Similar articles
-
Plasmodium vivax Pv12 B-cell epitopes and HLA-DRβ1*-dependent T-cell epitopes in vitro antigenicity.PLoS One. 2018 Sep 10;13(9):e0203715. doi: 10.1371/journal.pone.0203715. eCollection 2018. PLoS One. 2018. PMID: 30199554 Free PMC article.
-
Identification of HLA-A2 restricted CD8(+) T-lymphocyte responses to Plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria.Parasite Immunol. 2002 Mar;24(3):161-9. doi: 10.1046/j.1365-3024.2002.00449.x. Parasite Immunol. 2002. PMID: 12078650
-
Promiscuous T-cell epitopes of Plasmodium merozoite surface protein 9 (PvMSP9) induces IFN-gamma and IL-4 responses in individuals naturally exposed to malaria in the Brazilian Amazon.Vaccine. 2010 Apr 19;28(18):3185-91. doi: 10.1016/j.vaccine.2010.02.046. Epub 2010 Feb 26. Vaccine. 2010. PMID: 20189487 Free PMC article.
-
Development and longevity of naturally acquired antibody and memory B cell responses against Plasmodium vivax infection.PLoS Negl Trop Dis. 2024 Oct 24;18(10):e0012600. doi: 10.1371/journal.pntd.0012600. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39446698 Free PMC article. Review.
-
From a basic to a functional approach for developing a blood stage vaccine against Plasmodium vivax.Expert Rev Vaccines. 2020 Feb;19(2):195-207. doi: 10.1080/14760584.2020.1733421. Epub 2020 Feb 28. Expert Rev Vaccines. 2020. PMID: 32077349 Review.
Cited by
-
The persistence of naturally acquired antibodies and memory B cells specific to rhoptry proteins of Plasmodium vivax in patients from areas of low malaria transmission.Malar J. 2019 Nov 29;18(1):382. doi: 10.1186/s12936-019-3009-2. Malar J. 2019. PMID: 31783870 Free PMC article.
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
-
T Cell Peptides Derived from Invasive Stages of Schistosoma mansoni as Potential Schistosomiasis Vaccine.J Clin Med. 2021 Jan 24;10(3):445. doi: 10.3390/jcm10030445. J Clin Med. 2021. PMID: 33498845 Free PMC article.
-
Genetic sequence characterization and naturally acquired immune response to Plasmodium vivax Rhoptry Neck Protein 2 (PvRON2).Malar J. 2018 Oct 31;17(1):401. doi: 10.1186/s12936-018-2543-7. Malar J. 2018. PMID: 30382855 Free PMC article.
-
RON2, a novel gene in Babesia bigemina, contains conserved, immunodominant B-cell epitopes that induce antibodies that block merozoite invasion.Parasitology. 2019 Nov;146(13):1646-1654. doi: 10.1017/S0031182019001161. Epub 2019 Sep 13. Parasitology. 2019. PMID: 31452491 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

