Simple Methods and Rational Design for Enhancing Aptamer Sensitivity and Specificity
- PMID: 29868605
- PMCID: PMC5966647
- DOI: 10.3389/fmolb.2018.00041
Simple Methods and Rational Design for Enhancing Aptamer Sensitivity and Specificity
Abstract
Aptamers are structured nucleic acid molecules that can bind to their targets with high affinity and specificity. However, conventional SELEX (Systematic Evolution of Ligands by EXponential enrichment) methods may not necessarily produce aptamers of desired affinity and specificity. Thus, to address these questions, this perspective is intended to suggest some approaches and tips along with novel selection methods to enhance evolution of aptamers. This perspective covers latest novel innovations as well as a broad range of well-established approaches to improve the individual binding parameters (aptamer affinity, avidity, specificity and/or selectivity) of aptamers during and/or post-SELEX. The advantages and limitations of individual aptamer selection methods and post-SELEX optimizations, along with rational approaches to overcome these limitations are elucidated in each case. Further the impact of chosen selection milieus, linker-systems, aptamer cocktails and detection modules utilized in conjunction with target-specific aptamers, on the overall assay performance are discussed in detail, each with its own advantages and limitations. The simple variations suggested are easily available for facile implementation during and/or post-SELEX to develop ultrasensitive and specific assays. Finally, success studies of established aptamer-based assays are discussed, highlighting how they utilized some of the suggested methodologies to develop commercially successful point-of-care diagnostic assays.
Keywords: Kd; SELEX; affinity; aptamers; limit of detection; selectivity; sensitivity; specificity.
Figures


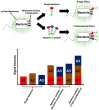
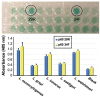

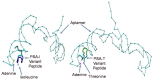
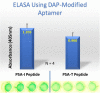
Similar articles
-
The Effects of SELEX Conditions on the Resultant Aptamer Pools in the Selection of Aptamers Binding to Bacterial Cells.J Mol Evol. 2015 Dec;81(5-6):194-209. doi: 10.1007/s00239-015-9711-y. Epub 2015 Nov 4. J Mol Evol. 2015. PMID: 26538121
-
An improved SELEX technique for selection of DNA aptamers binding to M-type 11 of Streptococcus pyogenes.Methods. 2016 Mar 15;97:51-7. doi: 10.1016/j.ymeth.2015.12.005. Epub 2015 Dec 8. Methods. 2016. PMID: 26678795
-
Selection of DNA aptamers for capture and detection of Salmonella Typhimurium using a whole-cell SELEX approach in conjunction with cell sorting.Appl Microbiol Biotechnol. 2013 Apr;97(8):3677-86. doi: 10.1007/s00253-013-4766-4. Epub 2013 Mar 14. Appl Microbiol Biotechnol. 2013. PMID: 23494620
-
SELEX Modifications and Bioanalytical Techniques for Aptamer-Target Binding Characterization.Crit Rev Anal Chem. 2016 Nov;46(6):521-37. doi: 10.1080/10408347.2016.1157014. Epub 2016 Mar 15. Crit Rev Anal Chem. 2016. PMID: 26980177 Review.
-
Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine.Int J Mol Sci. 2017 Oct 14;18(10):2142. doi: 10.3390/ijms18102142. Int J Mol Sci. 2017. PMID: 29036890 Free PMC article. Review.
Cited by
-
Computationally Designed Anti-LuxP DNA Aptamer Suppressed Flagellar Assembly- and Quorum Sensing-Related Gene Expression in Vibrio parahaemolyticus.Biology (Basel). 2022 Nov 1;11(11):1600. doi: 10.3390/biology11111600. Biology (Basel). 2022. PMID: 36358301 Free PMC article.
-
Surface Plasmon Resonance Aptasensors: Emerging Design and Deployment Landscape.Biosensors (Basel). 2025 Jun 4;15(6):359. doi: 10.3390/bios15060359. Biosensors (Basel). 2025. PMID: 40558441 Free PMC article. Review.
-
Advancements in Rapid and Affordable Diagnostic Testing for Respiratory Infectious Diseases: Evaluation of Aptamer Beacon Technology for Rapid and Sensitive Detection of SAR-CoV-2 in Breath Condensate.J Fluoresc. 2024 Nov;34(6):2629-2640. doi: 10.1007/s10895-023-03453-3. Epub 2023 Oct 21. J Fluoresc. 2024. PMID: 37864614
-
Fabrication and microfluidic analysis of graphene-based molecular communication receiver for Internet of Nano Things (IoNT).Sci Rep. 2021 Oct 1;11(1):19600. doi: 10.1038/s41598-021-98609-1. Sci Rep. 2021. PMID: 34599208 Free PMC article.
-
G-Quadruplex-Forming DNA Aptamers Inhibit the DNA-Binding Function of HupB and Mycobacterium tuberculosis Entry into Host Cells.Mol Ther Nucleic Acids. 2018 Dec 7;13:99-109. doi: 10.1016/j.omtn.2018.08.011. Epub 2018 Aug 22. Mol Ther Nucleic Acids. 2018. PMID: 30245472 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

