Endoscopic electrochemotherapy for esophageal cancer: a phase I clinical study
- PMID: 29868638
- PMCID: PMC5979192
- DOI: 10.1055/a-0590-4053
Endoscopic electrochemotherapy for esophageal cancer: a phase I clinical study
Abstract
Background and study aims: Esophageal cancer is on the rise in the western world and the disease has a poor 5-year survival prognosis below 20 %. Electrochemotherapy is a treatment where a chemotherapeutic drug is combined with locally applied electrical pulses, in order to increase the drug's cytotoxicity in malignant cells. This study presents the first results with electrochemotherapy treatment in esophageal cancer.
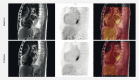
Patients and methods: In this first-in-human trial, six patients with advanced esophageal cancer were treated with electrochemotherapy using intravenous bleomycin. All side effects and adverse events (AEs) were registered and the patients were later evaluated with gastroscopy and 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging (18F-FDG PET/MRI).
Results: Treatment were well tolerated, main AEs being nausea, vomiting, oral thrush, pneumonia, retrosternal pain, fever, and hoarseness. No serious complications were observed. Five patients had a visual tumor response confirmed by gastroscopy. In two cases, these findings were confirmed with 18F-FDG PET/MRI as it revealed a reduction of total tumor mass.
Conclusion: Electrochemotherapy in patients with advanced esophageal cancer was conducted without major safety concerns. This study paves the way for larger studies, which may further elucidate response rates for and side effects of this new treatment.
Conflict of interest statement
Figures


References
-
- World Health Organization Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 2012. Available from:http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx[Accessed: 2017 May 01]
-
- Javle M, Ailawadhi S, Yang G Y et al.Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol. 2006;4:365–73, 379. - PubMed
-
- American Cancer Society Palliative therapy for cancer of the esophagusAvaible from:https://www.cancer.org/cancer/esophagus-cancer/treating/palliative-thera...[Accessed: 2017 May 17]
-
- Sersa G, Miklavcic D, Cemazar M et al.Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34:232–40. - PubMed
-
- Mir L M, Gehl J, Sersa G et al.Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer. 2006;4:14–25.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

