The Major Surface Glycoprotein of Pneumocystis murina Does Not Activate Dendritic Cells
- PMID: 29868908
- PMCID: PMC6173571
- DOI: 10.1093/infdis/jiy342
The Major Surface Glycoprotein of Pneumocystis murina Does Not Activate Dendritic Cells
Abstract
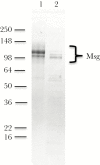
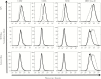
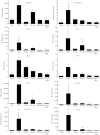
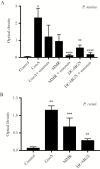
The major surface glycoprotein (Msg) is the most abundant surface protein among Pneumocystis species. Given that Msg is present on both the cyst and trophic forms of Pneumocystis and that dendritic cells play a critical role in initiating host immune responses, we undertook studies to examine activation of bone marrow-derived myeloid dendritic cells by Msg purified from Pneumocystis murina. Incubation of dendritic cells with Msg did not lead to increased expression of CD40, CD80, CD86, or major histocompatibility complex class II or to increased secretion of any of 10 cytokines. Microarray analysis identified very few differentially expressed genes. In contrast, lipopolysaccharide-activated dendritic cells had positive results of all of these assays. However, Msg did bind to mouse mannose macrophage receptor and human DC-SIGN, 2 C-type lectins expressed by dendritic cells that are important in recognition of pathogen-associated high-mannose glycoproteins. Deglycosylation of Msg demonstrated that this binding was dependent on glycosylation. These studies suggest that Pneumocystis has developed a mechanism to avoid activation of dendritic cells, potentially by the previously identified loss of genes that are responsible for the high level of protein mannosylation found in other fungi.
Figures

Similar articles
-
Cytokine responses to the native and recombinant forms of the major surface glycoprotein of Pneumocystis carinii.Clin Exp Immunol. 1997 Aug;109(2):255-60. doi: 10.1046/j.1365-2249.1997.4501348.x. Clin Exp Immunol. 1997. PMID: 9276520 Free PMC article.
-
The Trophic Life Cycle Stage of the Opportunistic Fungal Pathogen Pneumocystis murina Hinders the Ability of Dendritic Cells To Stimulate CD4+ T Cell Responses.Infect Immun. 2017 Sep 20;85(10):e00396-17. doi: 10.1128/IAI.00396-17. Print 2017 Oct. Infect Immun. 2017. PMID: 28694293 Free PMC article.
-
Additional C-type lectin receptors mediate interactions with Pneumocystis organisms and major surface glycoprotein.J Med Microbiol. 2021 Dec;70(12):001470. doi: 10.1099/jmm.0.001470. J Med Microbiol. 2021. PMID: 34889727 Free PMC article.
-
Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice.BMC Immunol. 2012 Jul 12;13:39. doi: 10.1186/1471-2172-13-39. BMC Immunol. 2012. PMID: 22788748 Free PMC article.
-
Characterization of pneumocystis major surface glycoprotein gene (msg) promoter activity in Saccharomyces cerevisiae.Eukaryot Cell. 2013 Oct;12(10):1349-55. doi: 10.1128/EC.00122-13. Epub 2013 Jul 26. Eukaryot Cell. 2013. PMID: 23893080 Free PMC article.
Cited by
-
Myeloid C-type lectin receptors that recognize fungal mannans interact with Pneumocystis organisms and major surface glycoprotein.J Med Microbiol. 2019 Nov;68(11):1649-1654. doi: 10.1099/jmm.0.001062. J Med Microbiol. 2019. PMID: 31609198 Free PMC article.
-
CLEC4A and CLEC12B C-type lectin receptors mediate interactions with Pneumocystis cell wall components.J Med Microbiol. 2023 Jun;72(6):001714. doi: 10.1099/jmm.0.001714. J Med Microbiol. 2023. PMID: 37294293 Free PMC article.
-
Retracing the evolution of Pneumocystis species, with a focus on the human pathogen Pneumocystis jirovecii.Microbiol Mol Biol Rev. 2024 Jun 27;88(2):e0020222. doi: 10.1128/mmbr.00202-22. Epub 2024 Apr 8. Microbiol Mol Biol Rev. 2024. PMID: 38587383 Free PMC article. Review.
-
Survey of the Transcription Factor Responses of Mouse Lung Alveolar Macrophages to Pneumocystis murina.Pathogens. 2021 May 8;10(5):569. doi: 10.3390/pathogens10050569. Pathogens. 2021. PMID: 34066663 Free PMC article.
-
The Contribution of Host Cells to Pneumocystis Immunity: An Update.Pathogens. 2019 Apr 19;8(2):52. doi: 10.3390/pathogens8020052. Pathogens. 2019. PMID: 31010170 Free PMC article. Review.
References
-
- Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 2009; 301:2578–85. - PubMed
-
- Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 2007; 5:298–308. - PubMed
-
- Bienvenu AL, Traore K, Plekhanova I, Bouchrik M, Bossard C, Picot S. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis 2016; 46:11–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

