Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease
- PMID: 29869016
- PMCID: PMC6132930
- DOI: 10.1007/s00535-018-1480-0
Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease
Abstract
Background: Selective patient recruitment can produce discrepancies between clinical trial results and real-world effectiveness.
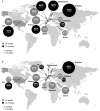
Methods: A systematic literature review and meta-analysis were conducted to assess vedolizumab real-world effectiveness and safety in patients with ulcerative colitis (UC) or Crohn's disease (CD). MEDLINE, MEDLINE In-Process, EMBASE, and Cochrane databases were searched for real-world studies of vedolizumab in adult patients with UC/CD reporting clinical response, remission, corticosteroid-free remission, UC/CD-related surgery or hospitalization, mucosal healing, or safety published from May 1, 2014-June 22, 2017. Response and remission rates were combined in random-effects meta-analyses.
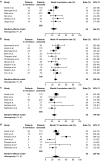
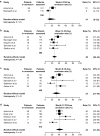
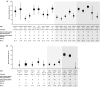
Results: At treatment week 14, 32% of UC patients [95% confidence interval (CI) 27-39%] and 30% of CD patients (95% CI 25-34%) were in remission; and at month 12, 46% for UC (95% CI 37-56%) and 30% for CD (95% CI 20-42%). For UC, the rates of corticosteroid-free remission were 26% at week 14 (95% CI 20-34%) and 42% at month 12 (95% CI 31-53%); for CD they were 25% at week 14 (95%, CI 20-31%) and 31% at month 12 (95%, CI 20-45%). At month 12, 33-77% of UC and 6-63% of CD patients had mucosal healing. Nine percent of patients reported serious adverse events.
Conclusions: Vedolizumab demonstrated real-world effectiveness in patients with moderate-to-severely active UC or CD, with approximately one-half and one-third of patients, respectively, in remission at treatment month 12. These findings are consistent with clinical trial data and support the long-term benefit-risk profile of vedolizumab.
Keywords: Crohn’s disease; Inflammatory bowel disease; Real-world effectiveness; Ulcerative colitis; Vedolizumab.
Conflict of interest statement
Stefan Schreiber has received honoraria from AbbVie, Celltrion, MSD, Pfizer, and Takeda. Axel Dignass has no conflict of interest. Laurent Peyrin-Biroulet has received lecture fees from MSD, Abbvie, Janssen, Takeda, Celltrion, Pfizer, BMS, Pharmacosmos, Shire, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, UCB Pharma, Hospira, Boehringer-Ingelheim, and Lilly. Greg Hather owns stock in Takeda Pharmaceuticals and is an employee of Takeda Global Research and Development. Dirk Demuth is an employee of Takeda International, UK Branch. Mahmoud Mosli has no conflict of interest. Rebecca Curtis is a former employee of Takeda International, UK Branch. Javaria Mona Khalid owns stock in Takeda Pharmaceuticals International and is an employee of Takeda International, UK Branch. Edward V. Loftus, Jr, has received research support from and has served as a consultant for Takeda.
Figures


References
-
- Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

