Tumor Formation of Adult Stem Cell Transplants in Rodent Arthritic Joints
- PMID: 29869062
- PMCID: PMC6279627
- DOI: 10.1007/s11307-018-1218-7
Tumor Formation of Adult Stem Cell Transplants in Rodent Arthritic Joints
Abstract
Purpose: While imaging matrix-associated stem cell transplants aimed for cartilage repair in a rodent arthritis model, we noticed that some transplants formed locally destructive tumors. The purpose of this study was to determine the cause for this tumor formation in order to avoid this complication for future transplants.
Procedures: Adipose-derived stem cells (ADSC) isolated from subcutaneous adipose tissue were implanted into 24 osteochondral defects of the distal femur in ten athymic rats and two immunocompetent control rats. All transplants underwent serial magnetic resonance imaging (MRI) up to 6 weeks post-transplantation to monitor joint defect repair. Nine transplants showed an increasing size over time that caused local bone destruction (group 1), while 11 transplants in athymic rats (group 2) and 4 transplants in immunocompetent rats did not. We compared the ADSC implant size and growth rate on MR images, macroscopic features, histopathologic features, surface markers, and karyotypes of these presumed neoplastic transplants with non-neoplastic ADSC transplants.
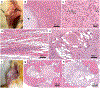
Results: Implants in group 1 showed a significantly increased two-dimensional area at week 2 (p = 0.0092), 4 (p = 0.003), and 6 (p = 0.0205) compared to week 0, as determined by MRI. Histopathological correlations confirmed neoplastic features in group 1 with significantly increased size, cellularity, mitoses, and cytological atypia compared to group 2. Six transplants in group 1 were identified as malignant chondrosarcomas and three transplants as fibromyxoid sarcomas. Transplants in group 2 and immunocompetent controls exhibited normal cartilage features. Both groups showed a normal ADSC phenotype; however, neoplastic ADSC demonstrated a mixed population of diploid and tetraploid cells without genetic imbalance.
Conclusions: ADSC transplants can form tumors in vivo. Preventive actions to avoid in vivo tumor formations may include karyotyping of culture-expanded ADSC before transplantation. In addition, serial imaging of ADSC transplants in vivo may enable early detection of abnormally proliferating cell transplants.
Keywords: Chondrosarcomas; Fibromyxoid sarcomas; Magnetic resonance imaging; Malignant tumors in vivo; Osteochondral transplants; Stem cell therapy.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures



References
-
- Stem cells entering clinical trials. http://clinicaltrials.gov/ct2/results?term=stem+cells (accessed 05/02/2012)
-
- Abudusaimi A, Aihemaitijiang Y, Wang YH, Cui L, Maimaitiming S, Abulikemu M (2011) Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. J Int Med Res 39:1852–1860 - PubMed
-
- Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT (2004) Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22:560–567 - PubMed
-
- Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y (2007) Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 28:5477–5486 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

