Evidence for a Sigmatropic and an Ionic Pathway in the Winstein Rearrangement
- PMID: 29870252
- PMCID: PMC6076873
- DOI: 10.1021/acs.joc.8b00961
Evidence for a Sigmatropic and an Ionic Pathway in the Winstein Rearrangement
Abstract
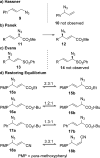
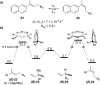
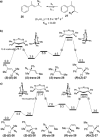
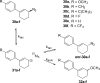
The spontaneous rearrangement of allylic azides is thought to be a sigmatropic reaction. Presented herein is a detailed investigation into the rearrangement of several allylic azides. A combination of experiments including equilibrium studies, kinetic analysis, density functional theory calculations, and selective 15N-isotopic labeling are included. We conclude that the Winstein rearrangement occurs by the assumed sigmatropic pathway under most conditions. However, racemization was observed for some cyclic allylic azides. A kinetic analysis of this process is provided, which supports a previously undescribed ionic pathway.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


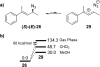
References
-
- Lutz RP. Catalysis of the Cope and Claisen Rearrangements. Chem. Rev. 1984;84:206–243.
-
- Nakai T, Mikami K. [2,3]-Wittig Sigmatropic Rearrangements in Organic Synthesis. Chem. Rev. 1986;86:885–902.
-
- Wilson SR, Price MF. The Ester Enolate Carroll Rearrangment. J. Org. Chem. 1984;49:722–725.
-
- Kantor SW, Hauser CR. Rearrangements of Benzyltrimethylammonium Ion and Related Quaternary Ammonium Ions by Sodium Amide Involving Migration into the Ring. J. Am. Chem. Soc. 1951;73:4122–4131.
-
- Evans DA, Andrews GC. Allylic Sulfoxides: Useful Intermediates in Organic Synthesis. Acc. Chem. Res. 1974;7:147–155.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

