Bone substitute made from a Brazilian oyster shell functions as a fast stimulator for bone-forming cells in an animal model
- PMID: 29870546
- PMCID: PMC5988300
- DOI: 10.1371/journal.pone.0198697
Bone substitute made from a Brazilian oyster shell functions as a fast stimulator for bone-forming cells in an animal model
Abstract
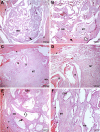
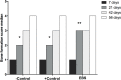
Despite their demonstrated biocompatibility and osteogenic properties, oyster shells have been reported as a potential alternative to other commonly used materials for bone substitution. This study evaluated whether an experimental bone substitute (EBS) made from a typical oyster shell of Northeastern Brazil (Crassostrea rhizophora) has effects on bone development using an animal model. Oysters were collected from a biologically assisted vivarium, and their inner layer was used for preparing an EBS. Chemical and surface characterization of EBS was performed using Individually Coupled Plasma Optical Emission Spectrometry (ICP-OES) and Scanning Electron Microscope (SEM), respectively. Seventy-two rats were randomly assigned to groups according to the treatment of bone defects created in the submandibular area: Negative Control (-C), Positive Control (+C; Bio-Oss®) and EBS. Euthanasia occurred at 7, 21, 42 and 56 days postoperatively. The bone pieces were stained with hematoxylin and eosin (H&E). The formation of bone tissue was evaluated histologically and histomorphometrically. Data were analyzed through the Kruskal-Wallis test and ANOVA considering a significant level of 5%. The main element found in EBS was calcium (71.68%), and it presented heterogeneity in the particle size and a porosity aspect at SEM analysis. Histological results revealed the absence of inflammatory cells in all groups, being that EBS presented the most accelerated process of bone formation with a statistically significant difference between this group and the +C and -C groups in the 21-day time-point (p < 0.05). After 21 days, the bone formation process was similar between all groups (p > 0.05), showing an immature lamellar bone pattern after 56 days of experimentation (p > 0.05). Within the limitations of this study, it was possible to conclude that EBS presented good biocompatibility and promoted fast stimulation for bone-forming cells in an animal model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Lang NP, Berglundh T, Heitz-Mayfield LJ, Pjetursson BE, Salvi GE, Sanz M. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int J Oral Maxillofac Implants. 2004;19(suppl):150–154. - PubMed
-
- Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P, Trisi P, et al. Implant success, survival, and failure: the international congress of oral implantologists (ICOI) Pisa consensus conference. Implant Dent. 2008;17:5–15. doi: 10.1097/ID.0b013e3181676059 - DOI - PubMed
-
- Penarrocha-Diago M, Penarrocha-Diago M, Zaragozí-Alonso R, Soto-Penaloza D. Consensus statements and clinical recommendations on treatment indications, surgical procedures, prosthetic protocols and complications following all-on-4 standard treatment. J Clin Exp Dent. 2017;9(5):e712–715. doi: 10.4317/jced.53759 - DOI - PMC - PubMed
-
- Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17(suppl 2):136–159. - PubMed
-
- Buser D, Dula K, Hirt HP. Lateral ridge augmentation using auto-grafts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996; 54:420–432. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

