Rhodanine derivatives as potent anti-HIV and anti-HSV microbicides
- PMID: 29870553
- PMCID: PMC5988308
- DOI: 10.1371/journal.pone.0198478
Rhodanine derivatives as potent anti-HIV and anti-HSV microbicides
Abstract
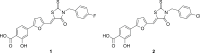
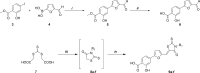
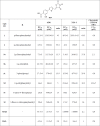
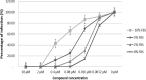
Although highly active antiretroviral therapies (HAART) remarkably increased life expectancy of HIV positive people, the rate of novel HIV-1 infections worldwide still represent a major concern. In this context, pre-exposure prophylaxis (PrEP) approaches such as vaginal microbicide gels topically releasing antiretroviral drugs, showed to have a striking impact in limiting HIV-1 spread. Nevertheless, the co-presence of other genital infections, particularly those due to HSV-1 or 2, constitute a serious drawback that strongly limits the efficacy of PrEP approaches. For this reason, combinations of different compounds with mixed antiviral and antiretroviral activity are thoroughly investigated Here we report the synthesis and the biological evaluation of a novel series of rhodanine derivatives, which showed to inhibit both HIV-1 and HSV-1/2 replication at nanomolar concentration, and were found to be active also on acyclovir resistant HSV-2 strains. The compounds showed a considerable reduction of activity in presence of serum due to a high binding to serum albumin, as determined through in vitro ADME evaluations. However, the most promising compound of the series maintained a considerable activity in gel formulation, with an EC50 comparable to that obtained for the reference drug tenofovir. Moreover, the series of compounds showed pharmacokinetic properties suitable for topical formulation, thus suggesting that the novel rhodanine derivatives could represent effective agents to be used as dual anti HIV/HSV microbicides in PrEP approaches.
Conflict of interest statement
The commercial affiliation to Lead Discovery Siena srl does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
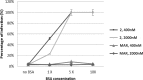
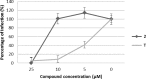
References
-
- World Health Organization. Global health sector strategy on sexually transmitted infections 2016–2021. 2016. Available from: http://apps.who.int/iris/bitstream/10665/246296/1/WHO-RHR-16.09-eng.pdf - PubMed
-
- Cates W Jr. After CAPRISA 004: time to re-evaluate the HIV lexicon. Lancet. 2010;376: 495–496. doi: 10.1016/S0140-6736(10)61200-7 - DOI - PubMed
-
- Karim QA, Kharsany AB, Frohlich JA, Baxter C, Yende N, Mansoor LE et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials. 2011;12: 67 doi: 10.1186/1745-6215-12-67 - DOI - PMC - PubMed
-
- Valley-Omar Z, Sibeko S, Anderson J, Goodier S, Werner L, Arnev L, et al. CAPRISA 004 tenofovir microbicide trial: no impact of tenofovir gel on the HIV transmission bottleneck. J Infect Dis. 2012;206: 35–40. doi: 10.1093/infdis/jis305 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

