RHO GTPases in cancer: known facts, open questions, and therapeutic challenges
- PMID: 29871878
- PMCID: PMC7615761
- DOI: 10.1042/BST20170531
RHO GTPases in cancer: known facts, open questions, and therapeutic challenges
Abstract
RHO GTPases have been traditionally associated with protumorigenic functions. While this paradigm is still valid in many cases, recent data have unexpectedly revealed that RHO proteins can also play tumor suppressor roles. RHO signaling elements can also promote both pro- and antitumorigenic effects using GTPase-independent mechanisms, thus giving an extra layer of complexity to the role of these proteins in cancer. Consistent with these variegated roles, both gain- and loss-of-function mutations in RHO pathway genes have been found in cancer patients. Collectively, these observations challenge long-held functional archetypes for RHO proteins in both normal and cancer cells. In this review, I will summarize these data and discuss new questions arising from them such as the functional and clinical relevance of the mutations found in patients, the mechanistic orchestration of those antagonistic functions in tumors, and the pros and cons that these results represent for the development of RHO-based anticancer drugs.
Keywords: CDC42; GTPase; RAC; RHO; RHO GAP; RHO GEF.
© 2018 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
The Author declares that there are no competing interests associated with this manuscript.
Figures
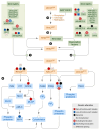
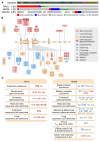

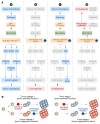
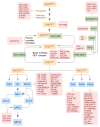
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

