The CCCH-type zinc finger transcription factor Zc3h8 represses NF-κB-mediated inflammation in digestive organs in zebrafish
- PMID: 29871925
- PMCID: PMC6078438
- DOI: 10.1074/jbc.M117.802975
The CCCH-type zinc finger transcription factor Zc3h8 represses NF-κB-mediated inflammation in digestive organs in zebrafish
Abstract
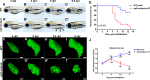
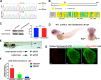
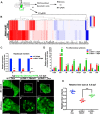
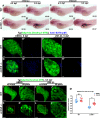
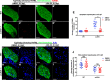
Degenerative diseases of organs lead to their impaired function. The cellular and molecular mechanisms underlying organ degeneration are therefore of great research and clinical interest but are currently incompletely characterized. Here, using a forward-genetic screen for genes regulating liver development and function in zebrafish, we identified a cq5 mutant that exhibited a liver-degeneration phenotype at 5 days postfertilization, the developmental stage at which a functional liver develops. Positional cloning revealed that the liver degeneration was caused by a single point mutation in the gene zc3h8 (zinc finger CCCH-type containing 8), changing a highly conserved histidine to glutamine at position 353 of the Zc3h8 protein. The zc3h8 mutation-induced liver degeneration in the mutant was accompanied by reduced proliferation, increased apoptosis, and macrophage phagocytosis of hepatocytes. Transcriptional profile analyses revealed up-regulation and activation of both proinflammatory cytokines and the NF-κB signaling pathway in the zc3h8 mutant. Suppression of NF-κB signaling activity efficiently rescued the proinflammatory cytokine response, as well as the inflammation-mediated liver degeneration phenotype of the mutant. Of note, the zc3h8 mutation-induced degeneration of several other organs, including the gut and exocrine pancreas, indicating that Zc3h8 is a general repressor of inflammation in zebrafish. Collectively, our findings demonstrate that Zc3h8 maintains organ homeostasis by inhibiting the NF-κB-mediated inflammatory response in zebrafish and that Zc3h8 dysfunction causes degeneration of multiple organs, including the liver, gut, and pancreas.
Keywords: NF-κ B (NF-κB); cell signaling; degeneration; digestive organ; inflammation; zebrafish; zinc finger.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Pack M., Solnica-Krezel L., Malicki J., Neuhauss S. C., Schier A. F., Stemple D. L., Driever W., and Fishman M. C. (1996) Mutations affecting development of zebrafish digestive organs. Development 123, 321–328 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

