Microtubule architecture in vitro and in cells revealed by cryo-electron tomography
- PMID: 29872007
- PMCID: PMC6096491
- DOI: 10.1107/S2059798318001948
Microtubule architecture in vitro and in cells revealed by cryo-electron tomography
Abstract
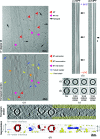
The microtubule cytoskeleton is involved in many vital cellular processes. Microtubules act as tracks for molecular motors, and their polymerization and depolymerization can be harnessed to generate force. The structures of microtubules provide key information about the mechanisms by which their cellular roles are accomplished and the physiological context in which these roles are performed. Cryo-electron microscopy allows the visualization of in vitro-polymerized microtubules and has provided important insights into their overall morphology and the influence of a range of factors on their structure and dynamics. Cryo-electron tomography can be used to determine the unique three-dimensional structure of individual microtubules and their ends. Here, a previous cryo-electron tomography study of in vitro-polymerized GMPCPP-stabilized microtubules is revisited, the findings are compared with new tomograms of dynamic in vitro and cellular microtubules, and the information that can be extracted from such data is highlighted. The analysis shows the surprising structural heterogeneity of in vitro-polymerized microtubules. Lattice defects can be observed both in vitro and in cells. The shared ultrastructural properties in these different populations emphasize the relevance of three-dimensional structures of in vitro microtubules for understanding microtubule cellular functions.
Keywords: cryo-electron tomography; lattice defects; microtubules; neurons; three-dimensional reconstruction.
open access.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

