GM-CSF targeted immunomodulation affects host response to M. tuberculosis infection
- PMID: 29872095
- PMCID: PMC5988704
- DOI: 10.1038/s41598-018-26984-3
GM-CSF targeted immunomodulation affects host response to M. tuberculosis infection
Abstract
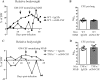
Host directed immunomodulation represents potential new adjuvant therapies in infectious diseases such as tuberculosis. Major cytokines like TNFα exert a multifold role in host control of mycobacterial infections. GM-CSF and its receptor are over-expressed during acute M. tuberculosis infection and we asked how GM-CSF neutralization might affect host response, both in immunocompetent and in immunocompromised TNFα-deficient mice. GM-CSF neutralizing antibodies, at a dose effectively preventing acute lung inflammation, did not affect M. tuberculosis bacterial burden, but increased the number of granuloma in wild-type mice. We next assessed whether GM-CSF neutralization might affect the control of M. tuberculosis by isoniazid/rifampicin chemotherapy. GM-CSF neutralization compromised the bacterial control under sub-optimal isoniazid/rifampicin treatment in TNFα-deficient mice, leading to exacerbated lung inflammation with necrotic granulomatous structures and high numbers of intracellular M. tuberculosis bacilli. In vitro, GM-CSF neutralization promoted M2 anti-inflammatory phenotype in M. bovis BCG infected macrophages, with reduced mycobactericidal NO production and higher intracellular M. bovis BCG burden. Thus, GM-CSF pathway overexpression during acute M. tuberculosis infection contributes to an efficient M1 response, and interfering with GM-CSF pathway in the course of infection may impair the host inflammatory response against M. tuberculosis.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Singhal, A. et al. Metformin as adjunct antituberculosis therapy. Science translational medicine6, 263ra159, 10.1126/scitranslmed.3009885 (2014). - PubMed
-
- O’Connor G, et al. Sharpening nature’s tools for efficient tuberculosis control: A review of the potential role and development of host-directed therapies and strategies for targeted respiratory delivery. Advanced drug delivery reviews. 2016;102:33–54. doi: 10.1016/j.addr.2016.04.024. - DOI - PubMed
-
- Yang Z, et al. How dormant is Mycobacterium tuberculosis during latency? A study integrating genomics and molecular epidemiology. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11:1164–1167. doi: 10.1016/j.meegid.2011.02.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

