Gut vagal sensory signaling regulates hippocampus function through multi-order pathways
- PMID: 29872139
- PMCID: PMC5988686
- DOI: 10.1038/s41467-018-04639-1
Gut vagal sensory signaling regulates hippocampus function through multi-order pathways
Abstract
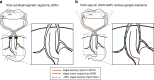
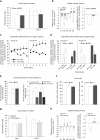
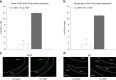
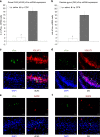
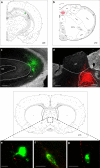
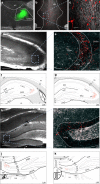
The vagus nerve is the primary means of neural communication between the gastrointestinal (GI) tract and the brain. Vagally mediated GI signals activate the hippocampus (HPC), a brain region classically linked with memory function. However, the endogenous relevance of GI-derived vagal HPC communication is unknown. Here we utilize a saporin (SAP)-based lesioning procedure to reveal that selective GI vagal sensory/afferent ablation in rats impairs HPC-dependent episodic and spatial memory, effects associated with reduced HPC neurotrophic and neurogenesis markers. To determine the neural pathways connecting the gut to the HPC, we utilize monosynaptic and multisynaptic virus-based tracing methods to identify the medial septum as a relay connecting the medial nucleus tractus solitarius (where GI vagal afferents synapse) to dorsal HPC glutamatergic neurons. We conclude that endogenous GI-derived vagal sensory signaling promotes HPC-dependent memory function via a multi-order brainstem-septal pathway, thereby identifying a previously unknown role for the gut-brain axis in memory control.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

