From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation
- PMID: 29872383
- PMCID: PMC5972222
- DOI: 10.3389/fnbeh.2018.00104
From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation
Abstract
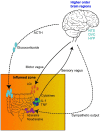
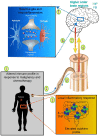
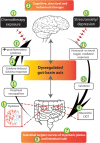
The central nervous system and gastrointestinal tract form the primary targets of chemotherapy-induced toxicities. Symptoms associated with damage to these regions have been clinically termed chemotherapy-induced cognitive impairment and mucositis. Whilst extensive literature outlines the complex etiology of each pathology, to date neither chemotherapy-induced side-effect has considered the potential impact of one on the pathogenesis of the other disorder. This is surprising considering the close bidirectional relationship shared between each organ; the gut-brain axis. There are complex multiple pathways linking the gut to the brain and vice versa in both normal physiological function and disease. For instance, psychological and social factors influence motility and digestive function, symptom perception, and behaviors associated with illness and pathological outcomes. On the other hand, visceral pain affects central nociception pathways, mood and behavior. Recent interest highlights the influence of functional gut disorders, such as inflammatory bowel diseases and irritable bowel syndrome in the development of central comorbidities. Gut-brain axis dysfunction and microbiota dysbiosis have served as key portals in understanding the potential mechanisms associated with these functional gut disorders and their effects on cognition. In this review we will present the role gut-brain axis dysregulation plays in the chemotherapy setting, highlighting peripheral-to-central immune signaling mechanisms and their contribution to neuroimmunological changes associated with chemotherapy exposure. Here, we hypothesize that dysregulation of the gut-brain axis plays a major role in the intestinal, psychological and neurological complications following chemotherapy. We pay particular attention to evidence surrounding microbiota dysbiosis, the role of intestinal permeability, damage to nerves of the enteric and peripheral nervous systems and vagal and humoral mediated changes.
Keywords: chemotherapy-induced cognitive impairment; chemotherapy-induced gut toxicity; gut-brain axis; microbiota; mucositis.
Figures

References
-
- Ahles T. A., Saykin A. J., McDonald B. C., Li Y., Furstenberg C. T., Hanscom B. S., et al. (2010). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J. Clin. Oncol. 28, 4434–4440. 10.1200/JCO.2009.27.0827 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

