Tryptophan immunoadsorption during pregnancy and breastfeeding in patients with acute relapse of multiple sclerosis and neuromyelitis optica
- PMID: 29872456
- PMCID: PMC5974561
- DOI: 10.1177/1756286418774973
Tryptophan immunoadsorption during pregnancy and breastfeeding in patients with acute relapse of multiple sclerosis and neuromyelitis optica
Abstract
Background: Up to every fourth woman with multiple sclerosis (MS) or neuromyelitis optica spectrum disorder (NMOSD) suffers a clinically relevant relapse during pregnancy. High doses of steroids bear some serious risks, especially within the first trimester of pregnancy. Immunoadsorption (IA) is an effective and more selective treatment option in disabling MS relapse than plasma exchange. Data on the use of IA during pregnancy and breastfeeding are scarce.
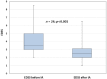
Methods: In this retrospective multicenter study, we analyzed the safety and efficacy of IA treatment in acute relapses during pregnancy or breastfeeding. The primary outcome parameter - change of acute relapse-related disability after IA - was assessed using Expanded Disability Status Scale (EDSS) and visual acuity (VA) measurements for patients with optic neuritis (ON).
Results: A total of 24 patients were analyzed, 23 with relapsing-remitting MS, and 1 with NMOSD. Twenty patients were treated with IA during pregnancy. Four patients received IA postnatally during the breastfeeding period. Treatment was started at a mean 22.5 [standard deviation (SD) 13.9] days after onset of relapse. Patients were treated with a series of 5.8 (mean, SD 0.7) IA treatments within 7-10 days. Sixteen patients received IA because of steroid-refractory relapse, eight were treated without preceding steroid pulse therapy. EDSS improved clinically relevant from 3.5 [median, interquartile range (IQR) 2] before IA to 2.5 (median, IQR 1.1) after IA, p < 0.001. In patients with ON, VA improved in four out of five patients. Altogether, in 83% of patients, a rapid and marked improvement of relapse-related symptoms was observed after IA with either a decrease of ⩾1 EDSS grade or improvement in VA ⩾20%. No clinically relevant side effect was reported in 138 IA treatments.
Conclusions: Tryptophan-IA was found to be effective and well tolerated in MS/NMOSD relapses, both as an escalation option after insufficient response to steroid pulse therapy and as first-line relapse treatment during pregnancy and breastfeeding.
Keywords: breastfeeding; immunoadsorption; multiple sclerosis; neuromyelitis optica spectrum disorder; plasma exchange; pregnancy; relapse; therapy.
Conflict of interest statement
Conflict of interest statement: F Hoffmann has received speaker honoraria and grant support from Bayer Vital, Biogen, DIAMED Medizintechnik, Merz, Genzyme, Grifols, Ipsen, Novartis and Teva. A Kraft has received grant support from Bayer Vital, Boehringer, Ipsen, Novartis and Pfizer. R Klingel and C Fassbender received research grants from Asahi Kasei Medical and DIAMED Medizintechnik. F Heigl received lecture fees from B Braun, Melsungen, DIAMED Medizintechnik and Fresenius Medical Care. J Koehler has reported a professional or personal relationship to Almirall, Bayer, Biogen Idec, Fresenius, Genzyme, Merck Serono, Novartis Pharma, Roche Pharma, Sanofi-Aventis and Teva. L Harms received grant support from Bayer Vital, Biogen, DIAMED Medizintechnik, Genzyme, Merz, Novartis, Roche and Teva. T Kümpfel has received travel expenses and speaker honoraria from Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, CLB Behring, Roche Pharma and Biogen, as well as grant support from Bayer-Schering AG, Novartis and Chugai Pharma. W. Köhler has received speaker honoraria and grant support from Bayer Vital, Biogen, DIAMED Medizintechnik, Merck Serono, Genzyme, Grifols, Ipsen, Novartis, Roche and Teva. A Bayas received personal compensation from Merck, Biogen, Bayer Vital, Novartis, Teva, Roche and Sanofi/Genzyme, and grants for congress trips and participation from Biogen, Teva, Novartis, Sanofi/Genzyme and Merck. G Ellrichmann has received grant support from Biogen, Novartis and Teva. T Slowinski received speaker fees and research grants from DIAMED Medizintechnik. K-H Henn received speaker honoraria from Genzyme, Merck Serono, Beratung Genzyme, Merck Serono, Novartis, Teva, and grant support from Teva, Biogen, Bayer, Merck Serono and Boehringer Ingelheim. S Schimrigk has received grant support and speaker honoraria from Bayer Vital, Bionorica research GmbH, Biogen, DIAMED Medizintechnik, Genzyme, Novartis, Pfizer and Teva. The following authors declare that there is no conflict of interest: J Weinmann-Menke, I Ayzenberg, E Mauch, H Weihprecht, S Ehrlich, C Beuker, H Fritz, M Brand, T Stiegler and J Galle.
Figures


References
-
- Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med 1998; 339: 285–291. - PubMed
-
- Benoit A, Durand-Dubief F, Amato M, et al. History of multiple sclerosis in 2 successive pregnancies: a French and Italian cohort. Neurology 2016; 87: 1360–1367. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

