Cascade Reaction by Chemo- and Biocatalytic Approaches to Obtain Chiral Hydroxy Ketones and anti 1,3-Diols
- PMID: 29872614
- PMCID: PMC5974551
- DOI: 10.1002/open.201800056
Cascade Reaction by Chemo- and Biocatalytic Approaches to Obtain Chiral Hydroxy Ketones and anti 1,3-Diols
Abstract
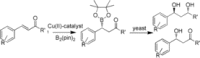
A chemo- and biocatalytic cascade approach was applied for the stereoselective synthesis of hydroxy ketones and the corresponding 1,3-diols. A new class of tridentate N,N,O ligands was used with copper(II) complexes for the asymmetric β-borylation of α,β-unsaturated compounds. The complex containing ligand L5 emerged as the best performer, and it gave the organoborane derivatives with good ee values. The corresponding keto-alcohol compounds were then bioreduced by yeasts. The biotransformation set up with Rhodotorula rubra allowed (R)-keto-alcohols and (S,S)-diols to be obtained with up to 99 % ee and up to 99 % de in favor of the anti enantiomers.
Keywords: biocatalysis; borylation; copper; reduction; yeast.
Figures

References
-
- None
-
- Rulli G., Duangdee N., Hummel W., Berkessel A., Gröger H., Eur. J. Org. Chem. 2017, 812–817;
-
- Sonoike S., Itakura T., Kitamura M., Aoki S., Chem. Asian J. 2012, 7, 64–74; - PubMed
-
- Vitale P., Perna F. M., Agrimi G., Scilimati A., Salomone A., Cardellicchio C., Capriati V., Org. Biomol. Chem. 2016, 14, 11438–11445; - PubMed
-
- El Gihani M. T., Williams J. M. J., Curr. Opin. Chem. Biol. 1999, 3, 11–15. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

