The potential of liquid biopsies for the early detection of cancer
- PMID: 29872715
- PMCID: PMC5871864
- DOI: 10.1038/s41698-017-0039-5
The potential of liquid biopsies for the early detection of cancer
Abstract
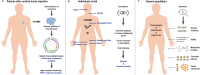
Precision medicine refers to the choosing of targeted therapies based on genetic data. Due to the increasing availability of data from large-scale tumor genome sequencing projects, genome-driven oncology may have enormous potential to change the clinical management of patients with cancer. To this end, components of tumors, which are shed into the circulation, i.e., circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), or extracellular vesicles, are increasingly being used for monitoring tumor genomes. A growing number of publications have documented that these "liquid biopsies" are informative regarding response to given therapies, are capable of detecting relapse with lead time compared to standard measures, and reveal mechanisms of resistance. However, the majority of published studies relate to advanced tumor stages and the use of liquid biopsies for detection of very early malignant disease stages is less well documented. In early disease stages, strategies for analysis are in principle relatively similar to advanced stages. However, at these early stages, several factors pose particular difficulties and challenges, including the lower frequency and volume of aberrations, potentially confounding phenomena such as clonal expansions of non-tumorous tissues or the accumulation of cancer-associated mutations with age, and the incomplete insight into driver alterations. Here we discuss biology, technical complexities and clinical significance for early cancer detection and their impact on precision oncology.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

