Organotropism: new insights into molecular mechanisms of breast cancer metastasis
- PMID: 29872722
- PMCID: PMC5871901
- DOI: 10.1038/s41698-018-0047-0
Organotropism: new insights into molecular mechanisms of breast cancer metastasis
Abstract
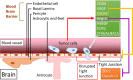
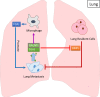
Metastasis accounts for 90% of breast cancer mortality. Despite the significant progress made over the past decade in cancer medicine our understanding of metastasis remains limited, therefore preventing and targeting metastasis is not yet possible. Breast cancer cells preferentially metastasize to specific organs, known as "organotropic metastasis", which is regulated by subtypes of breast cancer, host organ microenvironment, and cancer cells-organ interactions. The cross-talk between cancer cells and host organs facilitates the formation of the premetastatic niche and is augmented by factors released from cancer cells prior to the cancer cells' arrival at the host organ. Moreover, host microenvironment and specific organ structure influence metastatic niche formation and interactions between cancer cells and local resident cells, regulating the survival of cancer cells and formation of metastatic lesions. Understanding the molecular mechanisms of organotropic metastasis is essential for biomarker-based prediction and prognosis, development of innovative therapeutic strategy, and eventual improvement of patient outcomes. In this review, we summarize the molecular mechanisms of breast cancer organotropic metastasis by focusing on tumor cell molecular alterations, stemness features, and cross-talk with the host environment. In addition, we also update some new progresses on our understanding about genetic and epigenetic alterations, exosomes, microRNAs, circulating tumor cells and immune response in breast cancer organotropic metastasis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

