The proteome of neurofilament-containing protein aggregates in blood
- PMID: 29872749
- PMCID: PMC5986704
- DOI: 10.1016/j.bbrep.2018.04.010
The proteome of neurofilament-containing protein aggregates in blood
Abstract
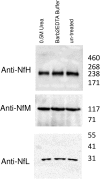
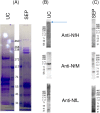
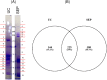
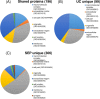
Protein aggregation in biofluids is a poorly understood phenomenon. Under normal physiological conditions, fluid-borne aggregates may contain plasma or cell proteins prone to aggregation. Recent observations suggest that neurofilaments (Nf), the building blocks of neurons and a biomarker of neurodegeneration, are included in high molecular weight complexes in circulation. The composition of these Nf-containing hetero-aggregates (NCH) may change in systemic or organ-specific pathologies, providing the basis to develop novel disease biomarkers. We have tested ultracentrifugation (UC) and a commercially available protein aggregate binder, Seprion PAD-Beads (SEP), for the enrichment of NCH from plasma of healthy individuals, and then characterised the Nf content of the aggregate fractions using gel electrophoresis and their proteome by mass spectrometry (MS). Western blot analysis of fractions obtained by UC showed that among Nf isoforms, neurofilament heavy chain (NfH) was found within SDS-stable high molecular weight aggregates. Shotgun proteomics of aggregates obtained with both extraction techniques identified mostly cell structural and to a lesser extent extra-cellular matrix proteins, while functional analysis revealed pathways involved in inflammatory response, phagosome and prion-like protein behaviour. UC aggregates were specifically enriched with proteins involved in endocrine, metabolic and cell-signalling regulation. We describe the proteome of neurofilament-containing aggregates isolated from healthy individuals biofluids using different extraction methods.
Keywords: Blood biomarkers; MS-based proteomics; NCH, neurofilament-containing hetero-aggregates; Neurofilaments; Nf, neurofilaments; NfH, neurofilament heavy chain; NfL, neurofilament light chain; NfM, neurofilament medium chain; PPS, pooled plasma sample; Protein aggregates; SEP, Seprion PAD-beads; Seprion PAD-Beads; UC, ultracentrifugation; Ultracentrifugation.
Figures
References
-
- Ross C.A., Poirier M.A. What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol. 2005;6(11):891–898. - PubMed
-
- Yang H., Hu H.Y. Sequestration of cellular interacting partners by protein aggregates: implication in a loss-of-function pathology. FEBS J. 2016;283(20):3705–3717. - PubMed
-
- Brangwynne C.P. Soft active aggregates: mechanics, dynamics and self-assembly of liquid-like intracellular protein bodies. Soft Matter. 2011;7(7):3052–3059.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

