The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age
- PMID: 29872772
- PMCID: PMC5986162
- DOI: 10.1016/j.heliyon.2018.e00644
The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age
Abstract
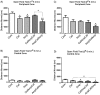
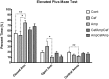
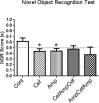
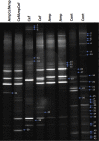
Recent studies carried on germ -free (GF) animal models suggest that the gut microbiota (GM) may play a role in the regulation of anxiety, mood, and cognitive abilities such as memory and learning processes. Consistently, any treatment disturbing the gut microbiota, including the overuse of antibiotics, may influence the brain functions and impact behavior. In the present study, to address this issue, two wide-spectrum antibiotics (ampicillin and cefoperazone, 1 g/l) were repeatedly applied throughout a 6-week period to initially 21-day-old male BALB/c mice. Antibiotics were administered separately or in a mixed fashion. On the completion of the antibiotic treatment, all mice were subjected to the behavioral tests. The serum levels of corticosterone and brain-derived neurotropic factor (BDNF) were assessed. Gut microbiota profiles were obtained by using denaturing gradient gel electrophoresis system, DGGE, from fecal samples. Ampicillin had a greater impact on both, gut microbiota composition and mice behavior compared to cefoperazone. All antibiotic-treated groups manifested a decrease in the locomotor activity and reduced recognition memory. However, the ampicillin-treated groups showed a higher anxiety level as assessed by the open field and the elevated plus maze tests and an increased immobility (behavioral despair) in the forced swim test. Obtained results evidently show that in mice, a repeated antibiotic treatment applied during adolescence, parallel to the changes in GM, affects locomotor activity, affective behavior and cognitive skills in young adults with ampicillin specifically enhancing anxiety- and depressive-like responses. Lower levels of serum BDNF were not associated with cognitive impairment but with changes in affective-like behaviors. Repeated administration of neither ampicillin nor cefoperazone affected basal serum corticosterone levels. This is one of the few studies demonstrating changes in a behavioral phenotype of young-adult subjects who were previously exposed to a repeated antibiotic treatment.
Keywords: Microbiology; Neuroscience.
Figures



References
-
- Bercik P., Collins S.M., Verdu E.F. Microbes and the gut-brain axis. Nat. Rev. Neurosci. 2012;12:453–466. - PubMed
-
- Burokas A., Moloney R.D., Dinan T.G., Cryan J.F. Microbiota regulation of the Mammalian gut- brain axis. Adv. Appl. Microbiol. 2015;91:1–626. - PubMed
-
- Cryan J.F., O'Mahony S.M. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol. Motil. 2011;23:187–192. - PubMed
-
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat. Rev. Neurosci. 2012;13:701–712. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

