Efficacy of capecitabine in patients with locally advanced or metastatic breast cancer with or without prior treatment with fluoropyrimidine: a retrospective study
- PMID: 29872875
- PMCID: PMC6060805
- DOI: 10.1007/s00280-018-3617-5
Efficacy of capecitabine in patients with locally advanced or metastatic breast cancer with or without prior treatment with fluoropyrimidine: a retrospective study
Abstract
Purpose: We conducted a retrospective study to assess the outcomes of capecitabine for advanced breast cancer (ABC) after perioperative fluoropyrimidines (FPs).
Methods: The charts of patients with ABC who received capecitabine between 2008 and 2016 at the National Cancer Center Hospital (Tokyo, Japan) were reviewed. Progression-free survival (PFS), overall survival (OS), tumor response, and adverse events (AEs) were compared between two groups: an FP group (prior perioperative FP use) and a non-FP group (no prior FP use).
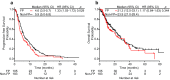
Results: Overall, 288 patients (FP n = 105; non-FP n = 183) were analyzed. The two groups had similar patient characteristics. The FP group had significantly poorer PFS than the non-FP group (multivariate hazard ratio [HR] 1.33; 95% confidence interval [CI] 1.02-1.73; p = 0.036), although the OS did not differ significantly between the groups (multivariate HR 1.00; 95% CI 0.67-1.50; p = 0.994). With different cut-off values (relapse-free interval [RFI] = 3, 4, and 5 years), multivariate HRs for PFS were 1.32-1.67 (short RFI), and 1.00-1.25 (long RFI). A trend for a larger HR in the FP group compared to the non-FP group with short RFI than in that with long RFI was also seen for OS. Response rate (RR) and disease control rate (DCR) did not differ significantly between the groups (RR in FP vs non-FP 13.8 vs 21.0%; p = 0.173; DCR 54.0 vs 59.9%; p = 0.418). No significant difference in AEs existed between the groups.
Conclusions: Extra caution is needed when capecitabine is considered for patients with ABC who used perioperative FP, especially those who had early recurrence.
Keywords: Breast neoplasms; Capecitabine; Efficacy; Fluoropyrimidine; Safety.
Conflict of interest statement
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval
This article does not contain any interventional studies with human participants performed by any of the authors. This study was approved by the ethics committee of each participating hospital and was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for this study.
Figures
Similar articles
-
Capecitabine combined with docetaxel versus vinorelbine followed by capecitabine maintenance medication for first-line treatment of patients with advanced breast cancer: Phase 3 randomized trial.Cancer. 2015 Oct 1;121(19):3412-21. doi: 10.1002/cncr.29492. Epub 2015 Jun 19. Cancer. 2015. PMID: 26096296 Clinical Trial.
-
[A multicenter clinical study for the comparison of S-1 versus capecitabine in the treatment of advanced breast cancer].Zhonghua Zhong Liu Za Zhi. 2017 Aug 23;39(8):607-612. doi: 10.3760/cma.j.issn.0253-3766.2017.08.009. Zhonghua Zhong Liu Za Zhi. 2017. PMID: 28835084 Chinese.
-
Hormonal therapy might be a better choice as maintenance treatment than capecitabine after response to first-line capecitabine-based combination chemotherapy for patients with hormone receptor-positive and HER2-negative, metastatic breast cancer.Chin J Cancer. 2016 Apr 25;35:39. doi: 10.1186/s40880-016-0101-7. Chin J Cancer. 2016. PMID: 27112139 Free PMC article.
-
Capecitabine: a review.Clin Ther. 2005 Jan;27(1):23-44. doi: 10.1016/j.clinthera.2005.01.005. Clin Ther. 2005. PMID: 15763604 Review.
-
Capecitabine in early breast cancer: A meta-analysis of randomised controlled trials.Eur J Cancer. 2017 May;77:40-47. doi: 10.1016/j.ejca.2017.02.024. Epub 2017 Mar 27. Eur J Cancer. 2017. PMID: 28355581 Review.
Cited by
-
Insight into Mantle Cell Lymphoma Pathobiology, Diagnosis, and Treatment Using Network-Based and Drug-Repurposing Approaches.Int J Mol Sci. 2024 Jul 2;25(13):7298. doi: 10.3390/ijms25137298. Int J Mol Sci. 2024. PMID: 39000404 Free PMC article.
References
-
- Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, Gralow J, Hortobagyi GN, Moy B, Yee D, Brundage SB, Danso MA, Wilcox M, Smith IE. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32(29):3307–3329. doi: 10.1200/JCO.2014.56.7479. - DOI - PMC - PubMed
-
- Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kolbl H, Janicke F, Kieback DG, Kuhn W, Schindler AE, Mohrmann S, Kaufmann M, Luck HJ. Multicenter phase II study of oral capecitabine (Xeloda®) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003;14(8):1227–1233. doi: 10.1093/annonc/mdg346. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous