Trastuzumab-induced cardiotoxicity and its risk factors in real-world setting of breast cancer patients
- PMID: 29872916
- PMCID: PMC11813419
- DOI: 10.1007/s00432-018-2682-9
Trastuzumab-induced cardiotoxicity and its risk factors in real-world setting of breast cancer patients
Abstract
Purpose: Cardiotoxicity is the most important side effect of trastuzumab treatment. Heart function monitoring is recommended during the treatment which has led to growing use of resources. The aim of this retrospective study was to determine the frequency and timing of trastuzumab cardiotoxicity and its risk factors in real-world setting.
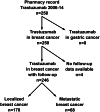
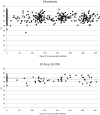
Methods: Single institute, retrospective collection of data on HER2+ breast cancer patients (n = 246) was carried out through a pharmacy search for patients who had received trastuzumab in 2006-2014. Clinical and pathological factors, treatment history, EF measurements, cardiac medications, cardiovascular disease history, cardiac symptoms, and survival data were collected from patient records.
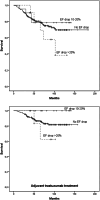
Results: 32 patients (13%) had EF decline ≥ 10%, eleven (4.5%) had EF decline ≥ 20% within 1 year after trastuzumab initiation, and trastuzumab was discontinued due to suspected cardiotoxicity in six patients (2.4%). 49 patients (19.9%) experienced symptoms related to cardiotoxicity during therapy, which accumulated among those with EF drop. Underlying cardiovascular diseases and multiple (≥ 2) cardiac medications were related to EF drop (≥ 20%) and trastuzumab discontinuation. Majority of EF drops (≥ 10%) and trastuzumab discontinuations were seen within 6months of trastuzumab initiation and recovery of EF drop to < 10% of the baseline was seen in most cases (62.5%). There was no statistically significant difference in the survival of patients according to EF drop.
Conclusions: Trastuzumab cardiotoxicity seems to accumulate among patients with underlying cardiac conditions. EF monitoring could be targeted to risk groups without compromising of the cardiac health or survival of HER2-positive breast cancer patients.
Keywords: Breast cancer; Cardiotoxicity; HER2 amplification; Trastuzumab.
Conflict of interest statement
Authors declare no conflict of interest. The study was funded by Oulu University Hospital, University of Oulu, and Finnish Cancer Society. According to national legislation, informed consent is not needed due to retrospective and non-interventional nature of the study. The opinions expressed in this article are the personal views of the author (OT) and may not be understood or quoted as being behalf or reflect the position of the Finnish Medicines Agency or European Medicines Agency.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

