Kushenol A and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens
- PMID: 29873272
- PMCID: PMC6009905
- DOI: 10.1080/14756366.2018.1477776
Kushenol A and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens
Abstract
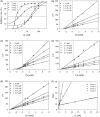
Tyrosinase is known for an enzyme that plays a key role in producing the initial precursor of melanin biosynthesis. Inhibition of the catalytic reaction of this enzyme led to some advantage such as skin-whitening and anti-insect agents. To find a natural compound with inhibitory activity towards tyrosinase, the five flavonoids of kushenol A (1), 8-prenylkaempferol (2), kushenol C (3), formononetin (4) and 8-prenylnaringenin (5) were isolated by column chromatography from a 95% methanol extract of Sophora flavescens. The ability of these flavonoids to block the conversion of L-tyrosine to L-DOPA by tyrosinase was tested in vitro. Compounds 1 and 2 exhibited potent inhibitory activity, with IC50 values less than 10 µM. Furthermore, enzyme kinetics and molecular docking analysis revealed the formation of a binary encounter complex between compounds 1-4 and the enzyme. Also, all of the isolated compounds (1-5) were confirmed to possess antioxidant activity.
Keywords: Fabaceae; Sophora flavescens; antioxidant; molecular docking; tyrosinase inhibitor.
Figures




References
-
- He X, Fang J, Huang L, et al. . Sophora flavescens Ait.: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol 2015;172:10–29. - PubMed
-
- Zhang W, Liu X, Fan H, et al. . Separation and purification of alkaloids from Sophora flavescens Ait. by focused microwave-assisted aqueous two-phase extraction coupled with reversed micellar extraction. Ind Crops Prod 2016;86:231–8.
-
- Liu G, Dong J, Wang H, et al. . Characterization of alkaloids in Sophora flavescens Ait. by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal Title 2011;54:1065–72. - PubMed
-
- Quang TH, Ngan NTT, Minh CV, et al. . Anti-inflammatory and PPAR transactivational properties of flavonoids from the roots of Sophora flavescens. Phytother Res 2013;27:1300–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
