Quality Measurement and Improvement Study of Surgical Coronary Revascularization: Medication Adherence (MISSION-2)
- PMID: 29873315
- PMCID: PMC6006808
- DOI: 10.4103/0366-6999.233767
Quality Measurement and Improvement Study of Surgical Coronary Revascularization: Medication Adherence (MISSION-2)
Abstract
Background: Secondary preventive therapies play a key role in the prevention of adverse outcomes after coronary artery bypass grafting (CABG). However, medication adherence after CABG is often poor, and conventional interventions for improving adherence have limited success. With increasing penetration of smartphones, health-related smartphone applications might provide an opportunity to improve adherence. Carefully designed trials are needed to provide reliable evidence for the use of these applications in patients after CABG.
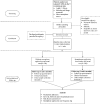
Methods: The Measurement and Improvement Studies of Surgical Coronary Revascularization: Medication Adherence (MISSION-2) study is a multicenter randomized controlled trial, aiming to randomize 1000 CABG patients to the intervention or control groups in a 1:1 ratio. We developed the multifaceted, patient-centered, smartphone-based Heart Health Application to encourage medication adherence in the intervention group through a health self-management program initiated during hospital admission for CABG. The application integrated daily scheduled reminders to take the discharge medications, cardiac educational materials, a dynamic dashboard to review cardiovascular risk factors and secondary prevention targets, and weekly questionnaires with interactive feedback. The primary outcome was secondary preventive medication adherence measured by the Chinese version of the 8-item Morisky Medication Adherence Scale at 6 months after randomization. Secondary outcomes included all-cause death, cardiovascular rehospitalization, and a composite of death, myocardial infarction, stroke, and repeat revascularization.
Discussion: Findings will not only provide evidence regarding the feasibility and effectiveness of the described intervention for improving adherence to CABG secondary preventive therapies but also explore a model for outpatient health self-management that could be translated to various chronic diseases and widely disseminated across resource-limited settings.
Trial registration: https://clinicaltrials.gov (NCT02432469).
冠脉搭桥质量改善研究:患者二级预防用药依从性研究(MISSION-2)摘要背景: 冠脉搭桥术后的二级预防药物治疗,对减少术后心血管不良事件发生至关重要。然而,既往研究显示患者二级预防用药依从性差,并且传统提高依从性的干预手段效果有限。智能手机在全球范围内的普及,将有望改变传统的医疗模式,低成本、高效率地提供更多诊疗机会,改善搭桥术后二级预防水平和患者预后。但目前亟需相关的高质量临床研究提供可靠的临床应用证据。 方法: MISSION-2研究是一个前瞻性、多中心、开放标签、随机对照的临床试验,计划入选1000例搭桥术后患者,采用1:1随机分组。根据“用户中心”设计思路,我们研发了手机应用“心健康”APP,对干预组患者从术后住院期间开始提供用药提醒,心脏术后健康宣教,心脏康复指导,交互式个体化生活方式建议。该研究的一级终点为术后6个月的依据8-MMAS量表测量的用药依从性。二级终点包括全因死亡、心血管再住院以及死亡、心梗、中风、再次血运重建的复合终点。 讨论: 该研究的结果,将为移动医疗技术在冠脉搭桥术后二级预防中应用的可行性和有效性提供可靠的临床证据。另外,试验的实施经验将为在医疗资源相对不足条件下,发展移动医疗技术改善慢病患者预后,探索患者院外自我管理新模式提供重要的借鉴。.
Keywords: Coronary Artery Bypass Grafting; Medication Adherence; Mobile Applications; Mobile Health; Secondary Prevention.
Conflict of interest statement
There are no conflicts of interest
Figures

Similar articles
-
Smartphone-based application to improve medication adherence in patients after surgical coronary revascularization.Am Heart J. 2020 Oct;228:17-26. doi: 10.1016/j.ahj.2020.06.019. Epub 2020 Jul 4. Am Heart J. 2020. PMID: 32745732 Clinical Trial.
-
Rationale and design of a randomized cluster trial to improve guideline-adherence of secondary preventive drugs prescription after coronary artery bypass grafting in China: Measurement and Improvement Studies of Surgical Coronary Revascularization: Secondary Prevention (MISSION-1) Study.Am Heart J. 2016 Aug;178:9-18. doi: 10.1016/j.ahj.2016.01.014. Epub 2016 Jan 25. Am Heart J. 2016. PMID: 27502847 Clinical Trial.
-
Statin Recapture Therapy before Coronary Artery Bypass Grafting Trial: Rationale and study design of a multicenter, randomized, double-blinded controlled clinical trial.Am Heart J. 2015 Jul;170(1):46-54, 54.e1-2. doi: 10.1016/j.ahj.2015.04.015. Epub 2015 Apr 17. Am Heart J. 2015. PMID: 26093863 Clinical Trial.
-
Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting in Patients With Left Main Coronary Artery Stenosis: A Systematic Review and Meta-analysis.JAMA Cardiol. 2017 Oct 1;2(10):1079-1088. doi: 10.1001/jamacardio.2017.2895. JAMA Cardiol. 2017. PMID: 28903139 Free PMC article.
-
Medical Therapy After CABG: the Known Knowns, the Known Unknowns, and the Unknown Unknowns.Cardiovasc Drugs Ther. 2024 Feb;38(1):141-149. doi: 10.1007/s10557-023-07444-1. Epub 2023 Mar 7. Cardiovasc Drugs Ther. 2024. PMID: 36881214 Review.
Cited by
-
Development and psychometric testing of the family caregiver self-efficacy scale for patients in the early post-coronary artery bypass grafting.PLoS One. 2025 Feb 12;20(2):e0314326. doi: 10.1371/journal.pone.0314326. eCollection 2025. PLoS One. 2025. PMID: 39937799 Free PMC article.
References
-
- WHO. Fact Sheet Number 310. The Top 10 Causes of Death. [Last accessed on 22 Aug 20]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/index.html .
-
- Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–210. doi: 10.1016/j.jacc.2011.08.009. - PubMed
-
- Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, et al. Secondary prevention after coronary artery bypass graft surgery: A scientific statement from the American Heart Association. Circulation. 2015;131:927–64. doi: 10.1161/cir.0000000000000182. - PubMed
-
- Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med. 2016;374:1954–64. doi: 10.1056/NEJMra1406944. - PubMed
-
- Liu JM, Ge L, Li J, Li X, Feng F, Zhang HB, et al. ACEI/ARB use among high risk patients with coronary heart disease in China: A cross-sectional study (In chinese) Chin J Cardiol. 2013;41:18–22. doi: 10.3760/cma.j.issn.0253-3758.2013.01.005. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

