Decoding the chemotactic signal
- PMID: 29873835
- PMCID: PMC6099250
- DOI: 10.1002/JLB.1MR0218-044
Decoding the chemotactic signal
Abstract
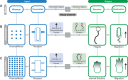
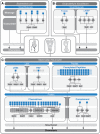
From an individual bacterium to the cells that compose the human immune system, cellular chemotaxis plays a fundamental role in allowing cells to navigate, interpret, and respond to their environments. While many features of cellular chemotaxis are shared among systems as diverse as bacteria and human immune cells, the machinery that guides the migration of these model organisms varies widely. In this article, we review current literature on the diversity of chemoattractant ligands, the cell surface receptors that detect and process chemotactic gradients, and the link between signal recognition and the regulation of cellular machinery that allow for efficient directed cellular movement. These facets of cellular chemotaxis are compared among E. coli, Dictyostelium discoideum, and mammalian neutrophils to derive organizational principles by which diverse cell systems sense and respond to chemotactic gradients to initiate cellular migration.
Keywords: G protein-coupled receptor; chemotaxis; communication theory; methyl-accepting chemotaxis protein receptor.
©2018 The Authors. Society for Leukocyte Biology Published by Wiley Periodicals, Inc.
Figures


References
-
- Nicholson DJ. Biological atomism and cell theory. Stud Hist Philos Biol Biomed Sci. 2010;41:202–211. - PubMed
-
- Bloemendal S, Kück U. Cell‐to‐cell communication in plants, animals, and fungi: a comparative review. Naturwissenschaften. 2013;100:3–19. - PubMed
-
- Steck K. Just follow your nose: homing by olfactory cues in ants. Curr Opin Neurobiol. 2012;22:231–235. - PubMed
-
- Hooke R. Micrographia. London: Royal Society; 1665.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

