Copper transporters and copper chaperones: roles in cardiovascular physiology and disease
- PMID: 29874110
- PMCID: PMC6139499
- DOI: 10.1152/ajpcell.00132.2018
Copper transporters and copper chaperones: roles in cardiovascular physiology and disease
Abstract
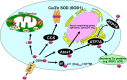
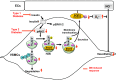
Copper (Cu) is an essential micronutrient but excess Cu is potentially toxic. Its important propensity to cycle between two oxidation states accounts for its frequent presence as a cofactor in many physiological processes through Cu-containing enzymes, including mitochondrial energy production (via cytochrome c-oxidase), protection against oxidative stress (via superoxide dismutase), and extracellular matrix stability (via lysyl oxidase). Since free Cu is potentially toxic, the bioavailability of intracellular Cu is tightly controlled by Cu transporters and Cu chaperones. Recent evidence reveals that these Cu transport systems play an essential role in the physiological responses of cardiovascular cells, including cell growth, migration, angiogenesis and wound repair. In response to growth factors, cytokines, and hypoxia, their expression, subcellular localization, and function are tightly regulated. Cu transport systems and their regulators have also been linked to various cardiovascular pathophysiologies such as hypertension, inflammation, atherosclerosis, diabetes, cardiac hypertrophy, and cardiomyopathy. A greater appreciation of the central importance of Cu transporters and Cu chaperones in cell signaling and gene expression in cardiovascular biology offers the possibility of identifying new therapeutic targets for cardiovascular disease.
Keywords: cardiovascular diseases; copper chaperones; copper homeostasis; copper transporters; vascular physiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

