Clinical Trial Participants' Views of the Risks and Benefits of Data Sharing
- PMID: 29874542
- PMCID: PMC6057615
- DOI: 10.1056/NEJMsa1713258
Clinical Trial Participants' Views of the Risks and Benefits of Data Sharing
Abstract
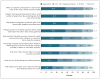
Background Sharing of participant-level clinical trial data has potential benefits, but concerns about potential harms to research participants have led some pharmaceutical sponsors and investigators to urge caution. Little is known about clinical trial participants' perceptions of the risks of data sharing. Methods We conducted a structured survey of 771 current and recent participants from a diverse sample of clinical trials at three academic medical centers in the United States. Surveys were distributed by mail (350 completed surveys) and in clinic waiting rooms (421 completed surveys) (overall response rate, 79%). Results Less than 8% of respondents felt that the potential negative consequences of data sharing outweighed the benefits. A total of 93% were very or somewhat likely to allow their own data to be shared with university scientists, and 82% were very or somewhat likely to share with scientists in for-profit companies. Willingness to share data did not vary appreciably with the purpose for which the data would be used, with the exception that fewer participants were willing to share their data for use in litigation. The respondents' greatest concerns were that data sharing might make others less willing to enroll in clinical trials (37% very or somewhat concerned), that data would be used for marketing purposes (34%), or that data could be stolen (30%). Less concern was expressed about discrimination (22%) and exploitation of data for profit (20%). Conclusions In our study, few clinical trial participants had strong concerns about the risks of data sharing. Provided that adequate security safeguards were in place, most participants were willing to share their data for a wide range of uses. (Funded by the Greenwall Foundation.).
Figures

Comment in
-
Green light for data sharing.Nat Med. 2018 Jul;24(7):898. doi: 10.1038/s41591-018-0122-7. Nat Med. 2018. PMID: 29988136 No abstract available.
References
-
- Institute of Medicine. Sharing clinical trial data: maximizing benefits, minimizing risk. Washington, DC: National Academies Press; 2015. - PubMed
-
- Zarin DA. Participant-level data and the new frontier in trial transparency. N Engl J Med. 2013;369:468–9. - PubMed
-
- Loder E. Sharing data from clinical trials: where we are and what lies ahead. BMJ. 2013;347:f4794. - PubMed
-
- Mello MM, Francer JK, Wilenzick M, Teden P, Bierer BE, Barnes M. Preparing for responsible sharing of clinical trial data. N Engl J Med. 2013;369:1651–8. - PubMed
-
- European Medicines Agency. Clinical data publication. ( http://www.ema.europa.eu/ema/?curl=pages/special_topics/general/general_...)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
